More Information
Submitted: August 14, 2023 | Approved: August 21, 2023 | Published: August 22, 2023
How to cite this article: Volkov AG, Hairston JS, Patel D, Sarkisov S. Cold Atmospheric Pressure Plasma Jet and Plasma Lamp Interaction with Plants: Electrostimulation, Reactive Oxygen and Nitrogen Species, and Side Effects. J Plant Sci Phytopathol. 2023; 7: 081-088.
DOI: 10.29328/journal.jpsp.1001110
Copyright License: © 2023 Volkov AG, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cold plasma; Phytopathology; Plasma phytoelectrophysiology; Reactive oxygen and nitrogen species; Plant signaling; Plasma agriculture
Cold Atmospheric Pressure Plasma Jet and Plasma Lamp Interaction with Plants: Electrostimulation, Reactive Oxygen and Nitrogen Species, and Side Effects
Alexander G Volkov1*, Jewel S Hairston1, Darayas Patel2 and Sergey Sarkisov3
1Department of Chemistry, Oakwood University, Huntsville, AL 35896, USA
2Department of Mathematics and Computer Science, Oakwood University, Huntsville, AL35896, USA
3SSS Optical Technologies LLC, 515 Sparkman Drive, Huntsville, AL 36816, USA
Alexander G Volkov, Department of Chemistry, Oakwood University, Huntsville, AL 35896, USA; Email: [email protected]
Cold atmospheric pressure plasma (CAPP) treatment is a highly effective method of protecting seeds, plants, flowers, and trees from diseases and infection and significantly increasing crop yields.
Here we found that cold atmospheric pressure He-plasma jet (CAPPJ) can also cause side effects and damage to plants if the plasma exposure time is too long. Reactive oxygen and nitrogen species (RONS), electromagnetic fields, and ultraviolet photons emitted by CAPPJ can cause both positive and negative effects on plants. CAPPJ can interact with biological tissue surfaces. The plasma lamp has no visible side effects on Aloe vera plants, cabbage, and tomatoes. A plasma lamp and a cold atmospheric pressure plasma He-jet cause strong electrical signaling in plants with a very high amplitude with frequencies equal to the frequency of plasma generation. The use of plasma lamps for electrostimulation of biological tissues can help to avoid side processes in biological tissues associated with the generation of RONS, UV photons, and direct interaction with cold plasma. CAPP technology can play an important role in agriculture, medicine, the food industry, chemistry, surface science, material science, and engineering applications without side effects if the plasma exposure is short enough.
CAPP treatment is a highly effective method of protecting bio-tissue from diseases and infection.
CAPP accelerates the imbibition and germination of seeds, plant growth, and nutrient absorption, activates enzymatic and ion channel activities [1-7], as well as promotes a significant increase in crop yields by up to 23% [2]. CAPP and plasma lamps can induce electroporation, corrugation, and morphological changes in the surfaces of seeds and biological tissues [4-6], as well as affect ion transport and bioelectrochemical characteristics of plant tissue [6,8-10].
There are also a few publications about side effects such as genotoxic effects, oxidation, and peroxidation of bio-tissue induced by direct treatment of bio-tissue with cold atmospheric pressure plasma [8,10-17].
A plasma lamp (also called a plasma ball or Tesla ball) is a clear borosilicate glass ball filled with a combination of noble gases at atmospheric pressure with an electrode in the center of the sphere. Plasma lamps were developed by Tesla [18] and their physical properties were investigated recently [19]. A plasma lamp was already used for the electrostimulation of seeds and plants [5,8,20]. Plasma lamps produce strong electromagnetic fields and oscillating visible light which can interact with a bio-tissue [19]. Plasma in plasma lamps is separated and covered by glass from a bio-tissue. It does not produce RONS outside of the glass.
The effect of electric fields on vegetation has been the subject of research since the eighteenth century [21-30]. Seed treatment with high-frequency electromagnetic fields using a plasma lamp can accelerate seed absorption, germination, and root growth without visible side effects [5,8,20]. Generated by plasma lamps or cold atmospheric pressure He-plasma jet (CAPPJ), high-frequency electromagnetic fields and photons can penetrate seed coats and modify their surface properties [5,8,20]. Treatment with a plasma lamp is not as effective for a harvest as treatment with a CAPPJ but usually does not generate side effects. Plasma lamps can be used in plasma agriculture to accelerate the germination of seeds, the growth of plant seedlings, and the corrugation of the surfaces of biological tissues without the side effects of reactive oxygen and nitrogen species (RONS) generated by plasma jets [5,8,20].
In this study, we tried to study possible side effects and electrical signaling in Aloe vera L., Brassica oleracea L., and Lycopersicon esculentum Mill. Plants caused by a cold atmospheric pressure He-plasma jet and a plasma ball. These plants are model objects for the study of electrical signaling and memory in plants [26,27,31-33].
In the present study, we tried to study possible side effects and electrical signaling in Aloe vera L., Brassica oleracea L., and Lycopersicon esculentum Mill. cv Cosmonaut Volkov plants caused These plants are model objects for the investigation of electrical signaling and memory in plants [26,27,31-33].
Plants
Forty Aloe vera L. plants were grown in clay pots with sterilized potting soil. Plants were exposed to a 12:12 hr light/ dark photoperiod at 21 oC. Aloe vera L. plants had 20 - 35 cm leaves. The soil around the Aloe vera L. plants was treated with water every week. Aloe vera plants were obtained from Bioelectrochemistry LLC (Madison, Al., USA).
Seedlings of Bonnie Hybrid Bonnie’s Best Cabbage (Brassica oleracea L.) were purchased from Bonnie Plant Farm (Union Spring, Al., USA). The soil around the cabbage plants was treated with water 3 times a week.
Forty tomatoes (Lycopersicon esculentum Mill. cv Cosmonaut Volkov) plants were grown in plastic pots with sterilized potting soil in a plant growth chamber (Environmental Corporation, USA). The soil around the tomato plants was treated with water every day. All measurements were performed on 21-to 28-day-old tomato plants. The seeds were purchased from various sources in Ukraine and Russia.
Irradiance was 850 μmol - 1100 μmol photons m-2s-1 PAR at plant level. All experiments were performed on healthy specimens. The relative humidity in the laboratory was kept at 45% - 50%.
Chemicals and test strips
Ozone test strips were purchased from Macherey-Nagel Company (Duren, Germany). These were used to determine the ozone concentration in the air near the surface of a plasma ball and under the plasma jet (Figure 2). Bottled ultra-high purity helium was purchased from Sexton Welding Supply (Huntsville, Al., USA).
Shielded extracellular electrodes
Teflon-coated silver wires (A-M Systems, Inc., Sequim, WA, USA) with a diameter of 0.2 mm were used for the preparation of non-polarizable Ag/AgCl electrodes. Identical shielded electrodes (Ag/AgCl or Cu) were used as working and reference electrodes for measurements of electrical potential differences in the plants.
ata acquisition
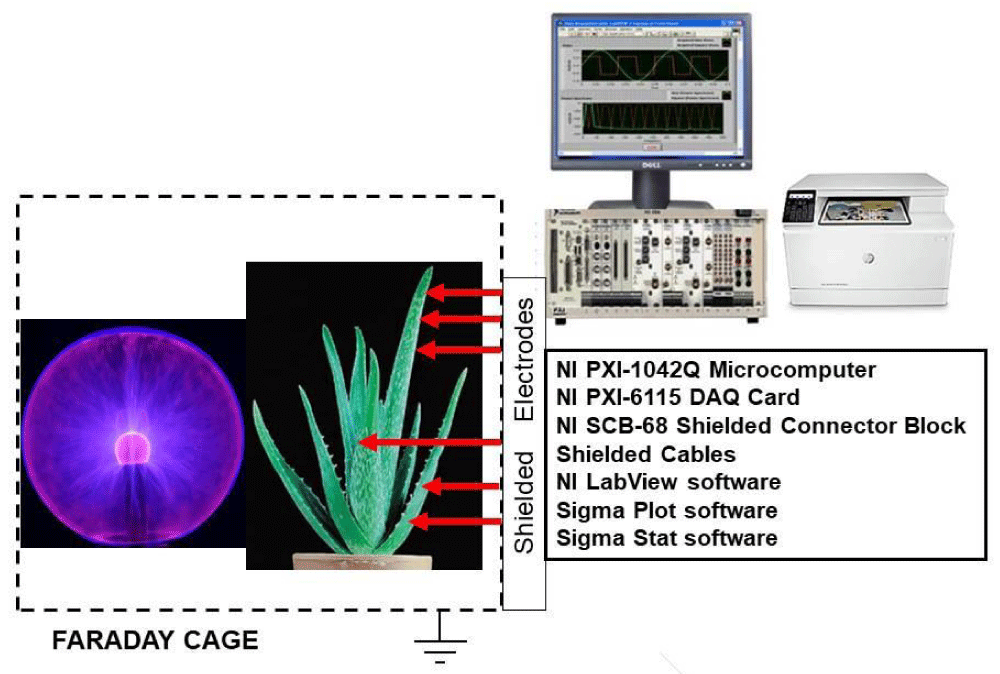
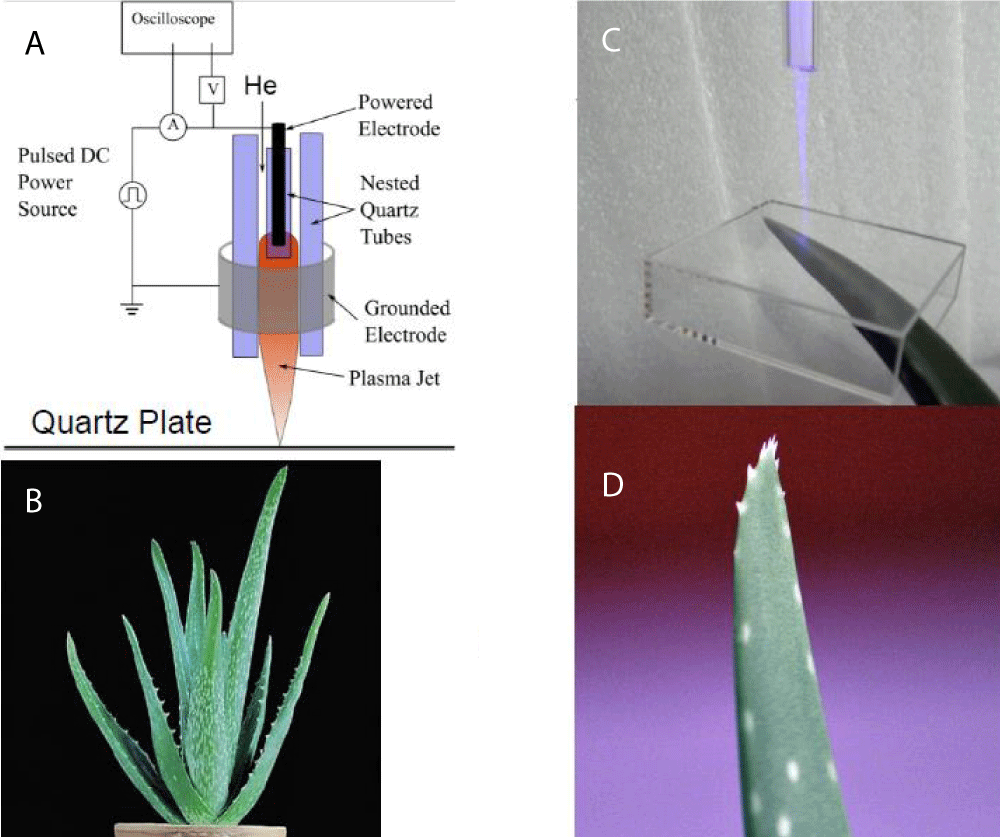
The experimental setup for tests using a plasma lamp is shown in Figure 1. High-speed data acquisition was performed using NI-PXI-1042Q microcomputers with simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 (National Instruments, Austin, TX, USA) interfaced through a NI-SCB-68 shielded connector block to Ag/AgCl electrodes. Data acquisition board NI-PXI-6115 (National Instruments, Austin, TX, USA) had a maximum sampling rate of 4,000,000 samples/s. The data acquisition board was connected to shielded electrodes.
Plasma ball and cold atmospheric pressure He-plasma jet
Common commercial plasma Nebula Plasma Ball (Figure 1) was used for electrostimulation of plants. The electromagnetic interference was measured with a CalTest CT2982B 10 kV high voltage divider probe (CalTest electronics, Yorba Linda, CA, USA) connected to a LeCroy wave runner LT322 oscilloscope (LeCroy, Chesnut Ridge, NY, USA).
The CAPPJ method was described earlier [6,7]. The system was operated with 8 kV pulse amplitude, 6 kHz pulse frequency, 1 µs pulse width, and a ~70 ns pulse rise and fall time. The entire system is placed in a metal enclosure to reduce electromagnetic interference.
FTIR spectra
FTIR spectra were recorded using a Thermo Scientific Nicolet ISS FT-IR spectrometer (ThermoFisher Scientific, Waltham, Massachusetts, USA). Reflectance spectra were recorded on a spectrophotometer ISR-2000 Plus with an integrating sphere (Shimadzu, Japan).
Temperature control
Digital laser temperature gun Etekcity laser grip 800 (Etekcity, Anaheim, CA, USA) was used to measure the temperature of the plasma jet, water, plants, and air. The temperature of the plasma jet, plants, and air during plasma treatment was 20 oC.
Statistics
All experimental results were reproduced at least 14 times using different plants of Aloe vera L., Brassica oleracea L. and Lycopersicon esculentum Mill. cv Cosmonaut Volkov.
Plasma lamps interaction with plants: Electro-stimulation
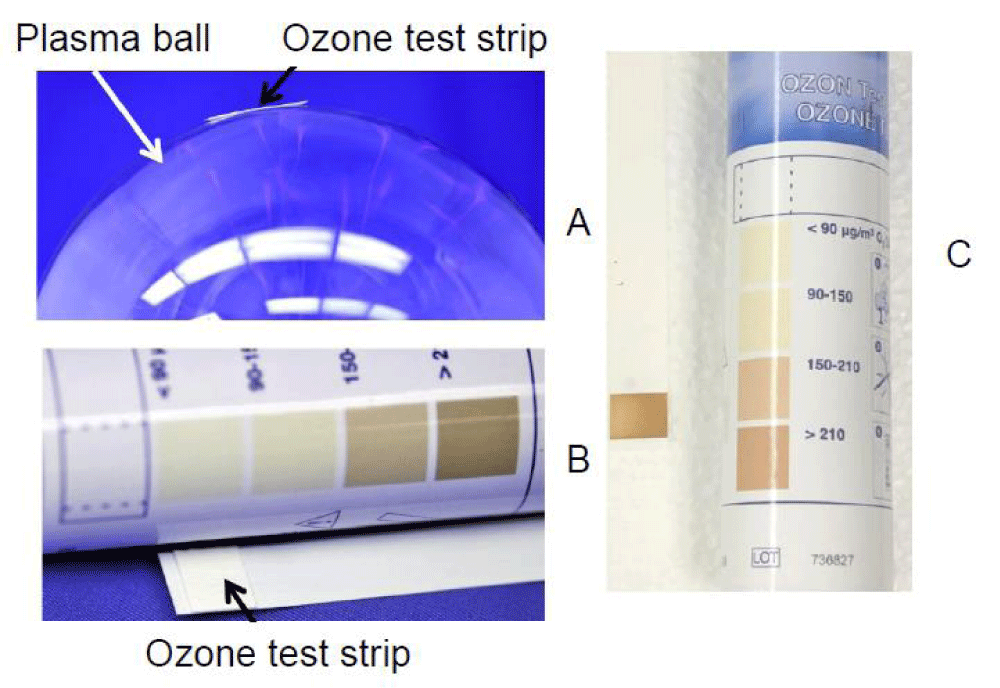
If a plant is placed on the outer surface of the ball or near the ball (Figure 1), the capacitive coupling can supply a high voltage load of up to several kV with a frequency of about 22 kHz. The electrical signal generated by the plasma ball is not limited to the glass sphere and propagates into the surrounding air in the form of electromagnetic interference (EMI). The amplitude of the electrical signal from the probe at a distance of 1 cm from the plasma ball was 628 V. UV-Vis radiation with a wavelength of more than 380 nm can penetrate through the glass wall of the plasma ball. While the plasma inside the ball is surrounded by the glass wall and does not produce a significant amount of RONS outside the lamp, high-frequency electromagnetic radiation propagates outside. The measurement of the ozone level on the surface of our plasma balls conducted with commercial ozone test strips exposed for 10 min did not show the formation of ozone in the air near the glass surface (Figure 2A,B). The ozone test strips, when placed under a cold He-plasma jet at a distance of 1 cm for 10 min, showed the production of ozone by the He-plasma jet at a concentration of more than 200 µg/m3 (Figure 2C).
Figure 1: Diagram of the experimental setup for detection of electrical plant responses induced with a plasma ball. A similar experimental setup was used for experiments with the Venus flytrap [8].
Figure 2: (A, B): Testing ozone production during 10 minutes exposure of to the ozone test strip near the surface of the plasma lamp. (C): Test of the ozone concentration after 10 min exposure to CAPPJ.
A monocot Aloe vera (L.) belongs to the Asphodelaceae (Liliaceae) family with crassulacean acid metabolism (CAM) and has been used for thousands of years in medicine, cosmetics, and as an ornamental plant such as was described in the Bible. Aloe vera stomata are open at night and closed during the day. The CO2 acquired by Aloe vera at night is temporarily stored as malic and other organic acids and is decarboxylated the following day to provide CO2 for fixation in the Calvin-Benson cycle behind closed stomata. Aloe vera is a model for the study of plant electrophysiology with crassulacean acid metabolism.
It is well known that DC and AC electrostimulation of plants can induce activation of ion channels and ion transport, gene expression, activation of enzymatic systems, electrical signaling, plant movements, enhanced wound healing, plant-cell damage, and plant growth (see for a review [26,27]). Recently, we analyzed the anisotropy and nonlinear properties of electrochemical circuits in the leaves of Aloe vera [31-33]. Along the conductive bundles, the behavior of Aloe vera leaves is highly nonlinear.
Electrostimulation by voltages with an amplitude higher than 2 V applied to the plant causes a drastic change in the leaf in the form of the initial input resistance drop. This change occurs in the conducting bundles and is probably related to the opening of voltage-gated ion channels in the Aloe vera leaf [31]. There is a strong electrical anisotropy of the Aloe vera leaf. In the direction across the conductive bundle, the behavior of the system is completely passive and linear like in a regular electric circuit with constant resistance. Conductance parallel to vascular bundles is two orders of magnitude higher than in the perpendicular direction.
The existence of electrical signaling in plants has been known for more than two centuries [21-25,28-30]. Direct measurements of plant electrical signaling induced by plasma jets turned out to be more complicated due to large electrical signals from plasma jets transmitted in plants.
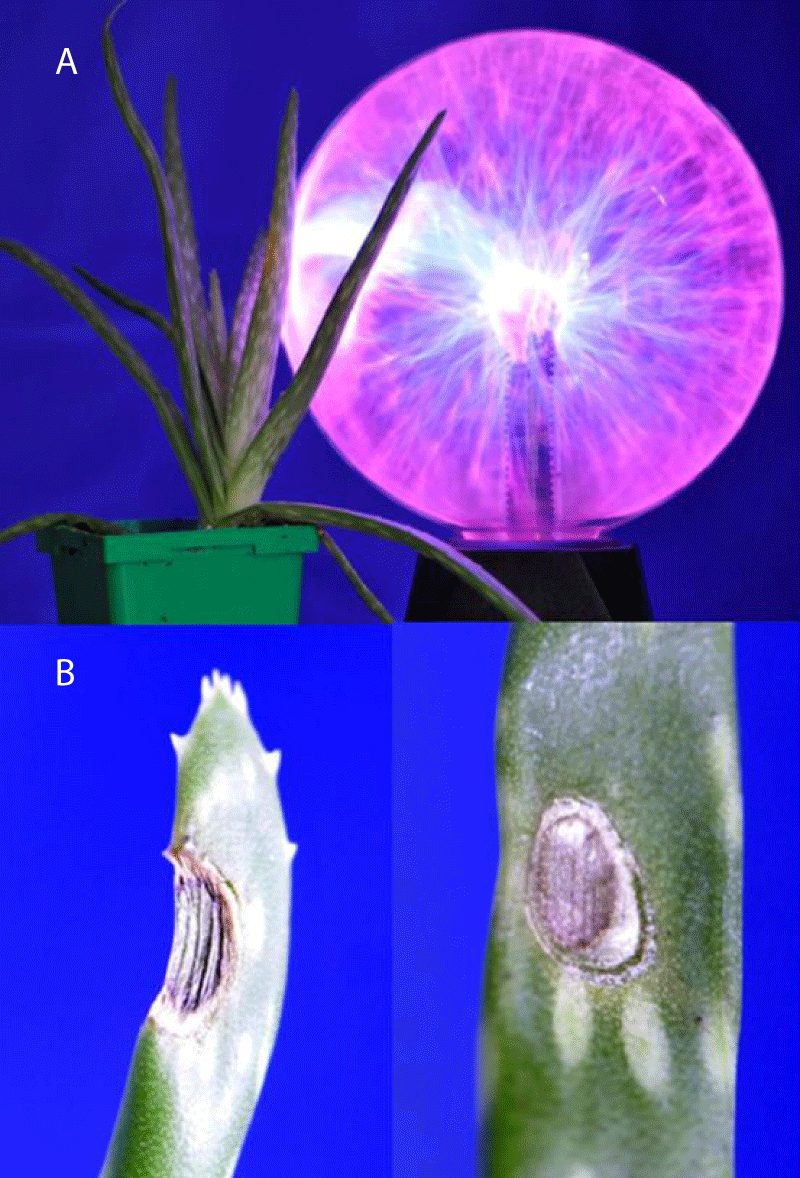
Prolonged treatment of Aloe vera leaves with the plasma ball does not cause any visible changes in the leaves during plasma treatment or after treatment (Figure 3A). The treatment of Aloe vera leaves with a cold atmospheric pressure He-plasma jet induces strong damage to leaves (Figure 3B).
Figure 3: (A) A 45 min treatment of Aloe vera with a plasma lamp does not induce damage to leaves. (B) Damage of Aloe vera leaves induced by 45 min treatment with He-plasma jet.
Electrostimulation of electrical networks in plants can induce electrotonic or action potentials propagating along their leaves and stems. Both action and electrotonic potentials play important roles in plant physiology and signal transduction between abiotic or biotic stress sensors and plant responses. It is well known that electrostimulation of plants can induce activation of ion channels, ion transport, plant-cell damage, enhanced wound healing, gene expression, enzymatic systems activation, electrical signaling, plant movements, and influence plant growth. Electrostimulation is an important tool for the evaluation of mechanisms of phytoactuators’ responses in plants without stimulation of abiotic or biotic stress phytosensors.
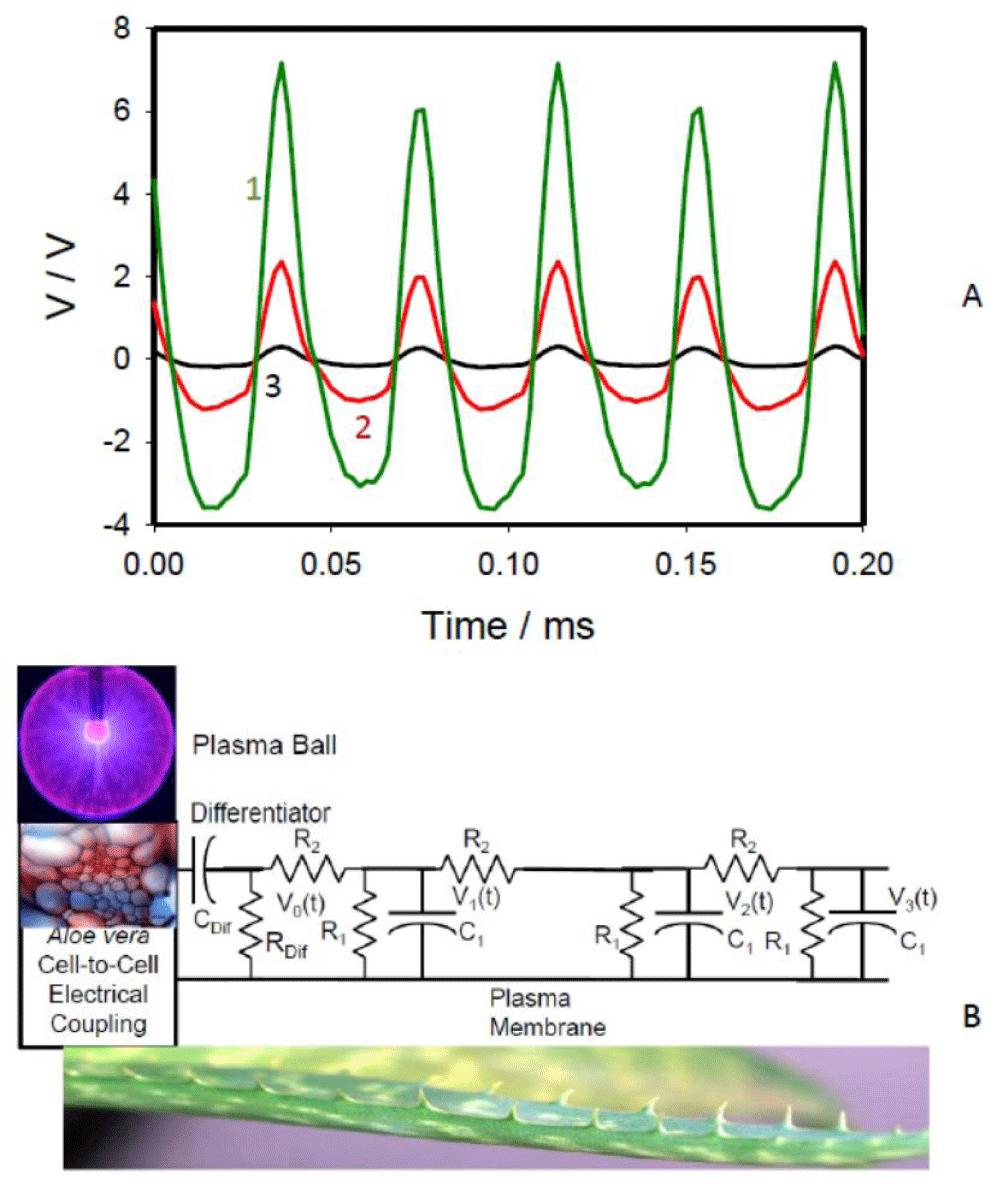
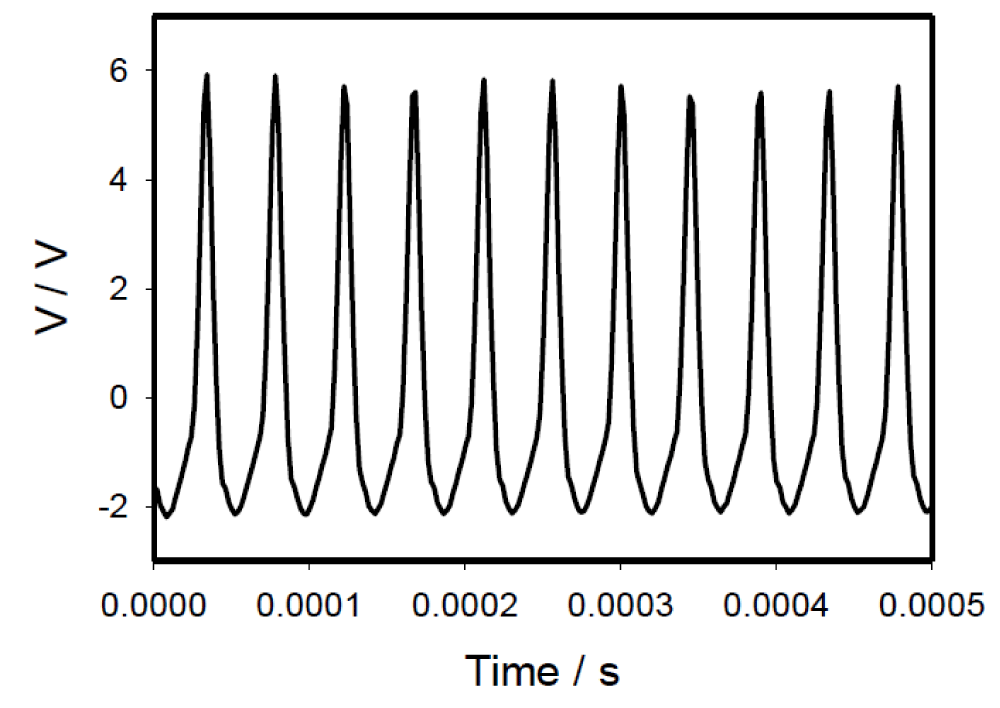
The plasma ball and CAPPJ caused strong electrical signaling along the leaves. The amplitude of the electric signal induced by the plasma lamp decreased with the distance in the leaf, but the frequency of 22 kHz was constant (Figure 4A). The amplitude of the electrical signal in the leaves of Aloe vera could reach several volts (Figure 4A). The propagation of the electric wave along the Aloe vera leaf can be illustrated by the equivalent electric diagram in Figure 4B. The mathematical model and experimental analysis of the electrotonic potential transduction in Aloe vera were presented earlier by Volkov and Shtessel [33].
Electrical signals can propagate along the plasma membrane over long and short distances in vascular bundles, plasmodesmata, and protoxylems. Electrotonic potentials in plants were discovered recently [31-33]. Electrostimulation of electrical circuits in Aloe vera induced electrotonic potentials with amplitude exponentially decreasing along a leaf or a stem.
The propagation of passive electrical signals in plant conductive bundles is usually interpreted in terms of the cable model [34]. The cable theory of the flow of electricity in a leaky cable was created by Lord Kelvin, who derived the equations to study the transatlantic telegraph cable. Hodgkin and Rushton [35] and Rall [36] applied the cable theory to passive electrical flow along membranes. Basic assumptions underlying the cable theory are as follows: (a) the electrotonic potential is due to the change in the membrane potential, which propagates in a cylinder with constant radius; (b) passive electrotonic current is ohmic in accord with the Ohm law; (c) electrotonic current divides between internal and membrane resistance; (d) membrane current is inversely proportional to membrane surface area; (e) axial current is inversely proportional to diameter.
If voltage-gated ion channels are closed and not involved in signal transduction along the plasma membrane, the transduction of electrotonic potentials can be described by the cable theory along a circuit consisting of capacitors C1 and resistors R1 and R2 (Figure 4B).
Figure 4: (A): Potential difference between Ag/AgCl electrodes in the Aloe vera leaf induced by the plasma lamp. The frequency of data acquisition scanning was 500,000 scan/s. Distances between the electrodes placed on the leaf were 4 cm. Distances between channels (pairs of Ag/AgCl electrodes) in the leaf and the plasma ball was 2 cm (1), 8 cm (2), and 14 cm (3). (B): The equivalent electrical scheme of passive electrical signal transmission along the Aloe vera leaf induced by the plasma lamp according to the theoretical model proposed by Volkov and Shtessel [33].
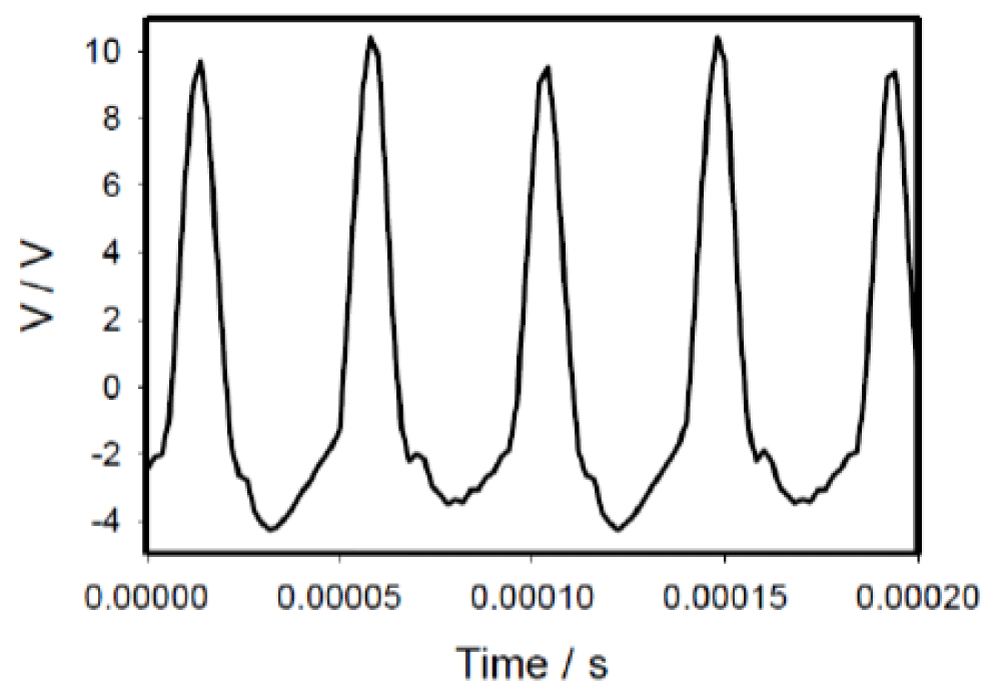
Plasma ball induced high-frequency electrical signaling in a cabbage leaf with an amplitude of several volts (Figure 5).
Figure 5: Electrical signals in the cabbage leaf induced by treatment with a plasma ball. The scanning frequency of data acquisition was 500,000 scan/s. The distance between the working and reference electrodes in the plant stem was 4 cm. The top leaf of the plant was in contact with the plasma ball. The amplitude of electrical signals was about 14 V (mean 13.91 V, median 14.30 V, std. dev. 0.85 V, std. err. 0.27 V, n = 100).
The frequency of the electrical wave corresponds to the frequency of the electromagnetic field in the plasma ball.
Electrical signals in tomato plants induced by the plasma ball are shown in Figure 6. These results of electrical waves in tomato plants (Figure 6) are very similar to the electrical waves in cabbage (Figure 5) and Aloe vera leaves (Figure 4).
Cold atmospheric pressure plasma He-jet interaction with plants
Direct treatment of plants with CAPPJ can damage their tissue (Figure 3B). It can be the effect of RONS, UV-Vis light, and electromagnetic fields produced by the plasma. A thin quartz plate was inserted between the plasma jet and a leaf of Aloe vera in control experiments. UV-Vis light and electromagnetic fields can penetrate through the quartz plate without visibly damaging the leaf (Figure 7). This means that RONS was responsible for damaging the plant tissue in Aloe vera (Figure 3B).
Figure 6: Electrical signals in tomato plant (Lycopersicon esculentum Mill. Cv Cosmonaut Volkov) induced by treatment with the plasma lamp. The frequency of data acquisition scanning was 1000,000 scan/s. The distance between the working and reference electrodes was 3 cm. The amplitude of electrical signals was about 7.87 V (mean 7.87 V, median 7.85 V, std. dev. 0.13 V, std. err. 0.03 V, n = 100).
Figure 7: Treatment of Aloe vera leaf with a cold He-plasma jet separated by a thin quartz plate. (A) Cold atmospheric pressure He-plasma jet above a quartz plate; (B) Aloe vera plant; (C) Treatment of Aloe vera leaf with cold He-plasma jet covered by a thin quartz plate from an optical cuvette; (D) Aloe vera leaf after 100 minutes of treatment in the setup shown on the panel (C).
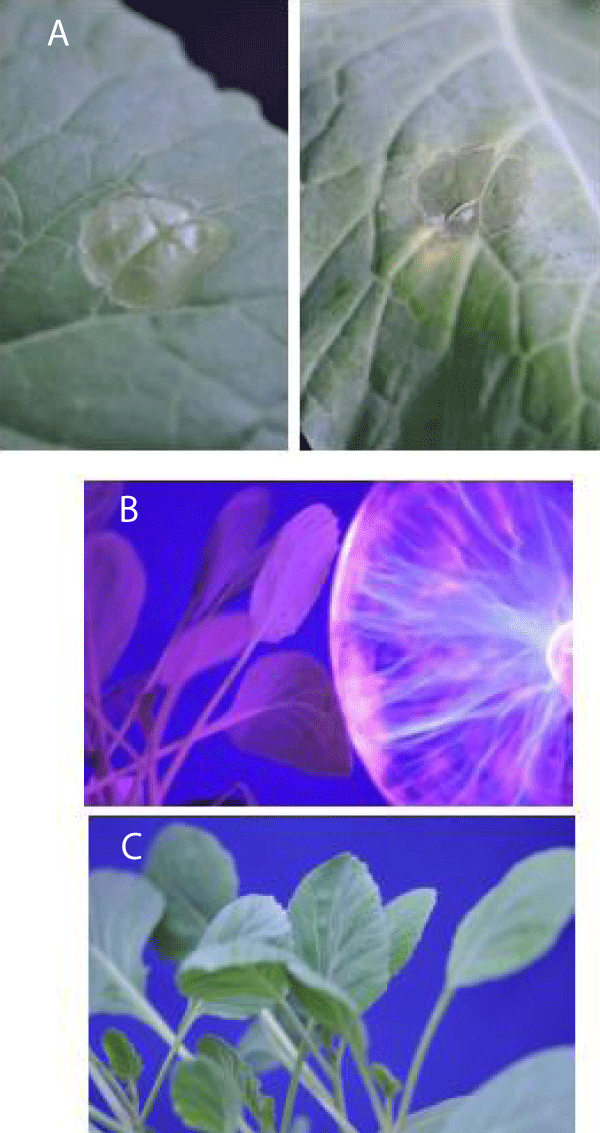
The treatment of cabbage leaves with a cold He-plasma jet caused visible damage around the place of contact of the plasma with the leaf (Figure 8A). The plasma ball treatment does not cause visible damage to the cabbage leaf (Figure 8B,C).
Figure 8: (A): Bonnie hybrid cabbage leaves after 10 min treatment with cold atmospheric pressure He-plasma jet. (B) and (C): Bonnie hybrid cabbage leaves during (B) and after (C) 75 min treatment with a plasma ball. Results were reproduced 16 times using different plants.
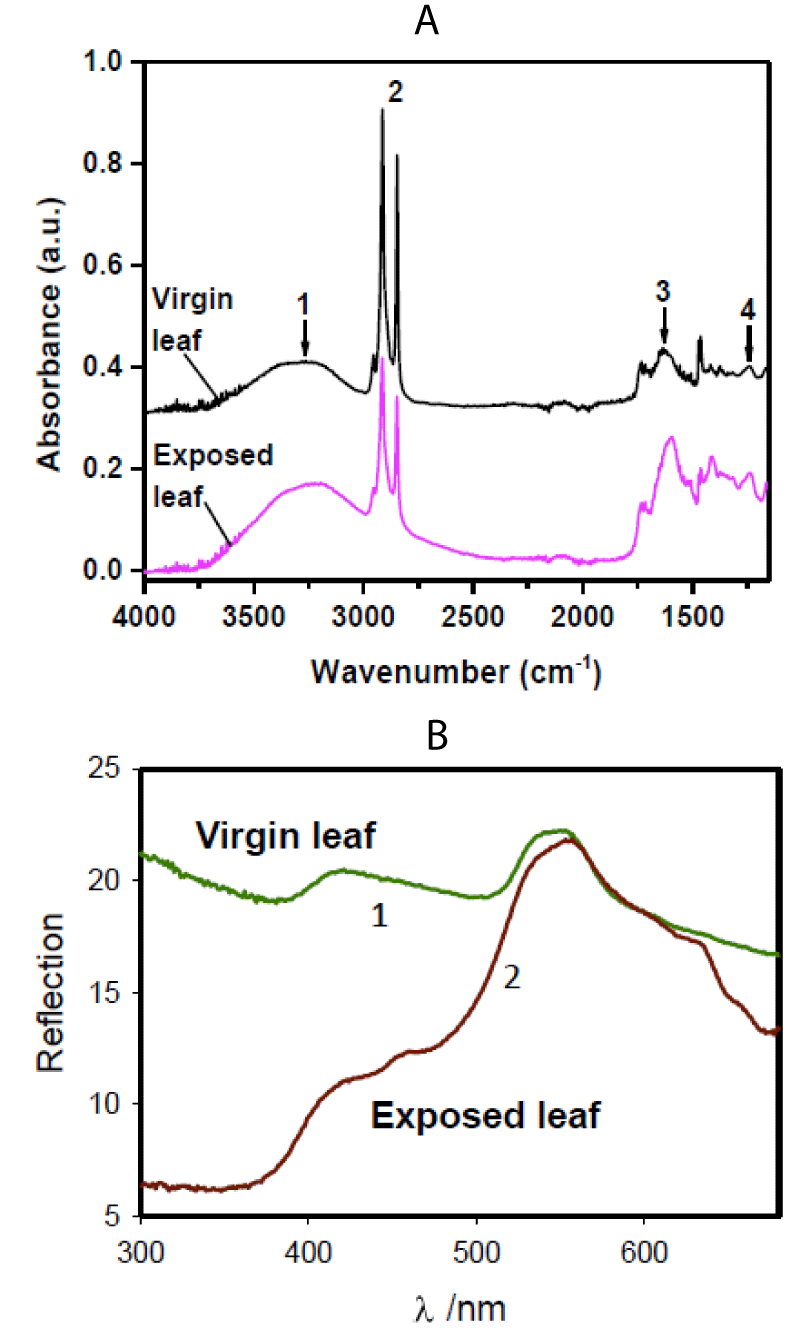
The FTIR and optical diffusive reflectance spectra (Figure 9) show the difference between untreated and treated cabbage leaves when the treatment was done with the He-plasma jet for 10 min. The FTIR spectra have an absorption maximum of 1 (Figure 9A) at about 3200 cm−1 which can be attributed to the phenol O-H stretching groups [37]; peak 2 is from the resonance groups of the C-H aliphatic (between 2850 and 3000 cm−1) [37]; peak 3 at 1603 cm−1 is from the resonance groups of the aromatic C=C [37]; and peak 4 at 1246 cm-1 refers to the C-O stretching from hemicellulose and lignin [37].
Figure 9: (A): FTIR spectra of Bonnie hybrid cabbage leaf before and after 10 min treatment with cold atmospheric pressure He-plasma jet. (B): Optical diffusion reflectance spectra of Bonnie hybrid cabbage leaf before and after 10 min treatment with CAPPJ. Results were reproduced 14 times using different plants.
Reflectance spectra of Bonnie hybrid cabbage leaf before and after 10 min treatment with cold atmospheric pressure He-plasma jet have a very significant difference between 300 nm and 550 nm (Figure 9B). This is most likely caused by the degradation of chlorophyll by reactive oxygen species in the brown spots of leaves treated with the cold He-plasma jet (Figure 8A).
Most publications and patents about the effect of cold plasma on plants focus on a significant increase in crop yield and plant sustainability [2,3,37]. The new terms “plasma seeds” and “plasma agriculture” have been widely used in the last 25 years. Cold plasma can protect surfaces of a bio-tissue against bacteria, viruses, fungi, and mold [38-40]. Here we found for the first time that cold atmospheric pressure He-plasma jet (CAPPJ) can also cause side effects and damage to plants. UV-Vis radiation, high frequency strong electromagnetic field, RONS, ions, and free electrons from plasma can also generate side effects (Figures 3-8) and changes in the composition of plants and fruits [14,41,42]. Reactive oxygen species can induce plant cell death [43-45]. Ozone can induce necrosis and increase peroxidase activity [46]. The development of side effects depends on the duration of plasma treatment. Plasma disinfects many bacteria in half a minute [39], so the optimal time for processing seeds and plants with cold atmospheric pressure He-plasma jet is in the range of 10 to 60 seconds, although this time also depends on the composition of the gas phase used to produce plasma. We found that the electrostimulation of plants by plasma lamps can help to avoid side processes in biological tissues associated with the generation of RONS.
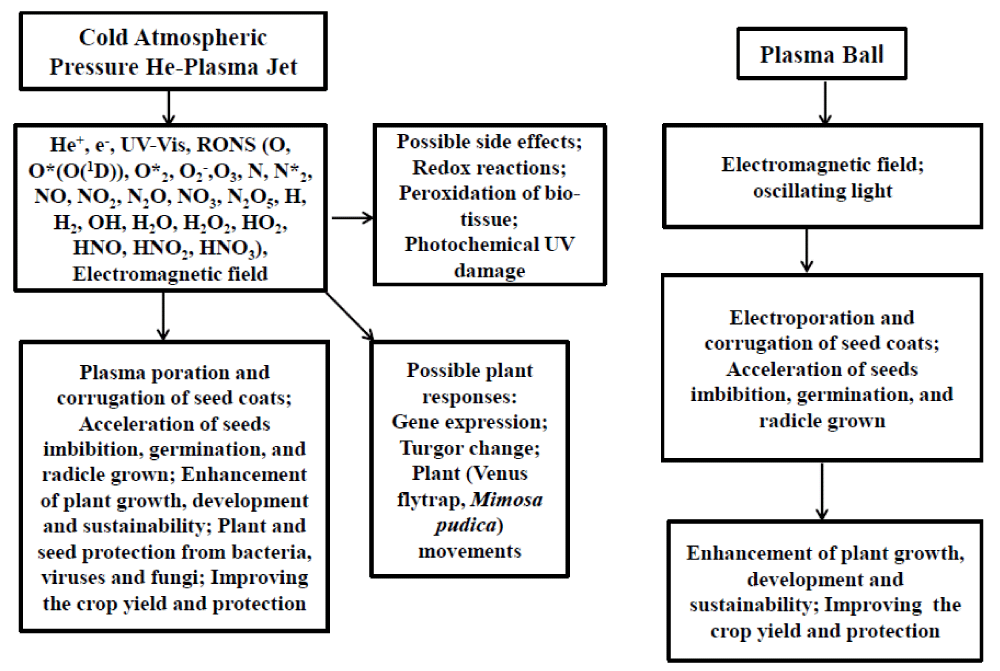
This article gives a new insight into the possible side effects of cold plasma interactions with plants. Scheme 1 shows mechanisms of interaction of cold atmosphere pressure He-plasma jet and/or plasma lamps with seeds and plants. It is known that CAPP in the air produces RONS at room temperature. Reactive oxygen and nitrogen species play important roles in plant physiology and agriculture. They can be very toxic to biological tissue and can selectively kill bacteria, fungi, and viruses. At the same time, RONS are useful companions of plant developmental processes and the activation of ion channels. RONS are involved in signal transduction, interactions with ion channels, and cell death. The specific biological response of a plant to RONS depends on the chemical identity of the RONS, the intensity of the signal, sites of production, the plant developmental stage, and interactions with other signaling molecules such as nitric oxides, hydrogen peroxide, ozone, and nitic acid. Cold plasma can affect ion transport and bioelectrochemical characteristics of plant tissue. Generated by the cold atmospheric pressure He-plasma jet reactive oxygen and nitrogen species, UV-Vis photons, and high-frequency strong electromagnetic fields with amplitudes of a few kV can interact with plants. Here we found that RONS produced by CAPPJ can also cause side effects and damage to plants if the plasma exposure is long enough. The plasma lamp has no visible side effects on Aloe vera, cabbage, and tomato plants, but induces electrical waves with very high amplitude in plants. The plasma ball creates high-frequency electromagnetic fields that can be used for electroporation and corrugation of biological tissues. Understanding the mechanisms of plasma interactions with seeds and plants can contribute to the development of plasma-based technology to control plant developmental, increase yield and growth rates, and protect from pathogens. Low-temperature atmospheric pressure plasma can play an important role in agriculture, medicine, food processing, disinfection and sterilization, and biophysical and biochemical applications.
Scheme 1: Interaction of cold atmospheric pressure He-plasma jet and the plasma ball with a plant and seed tissue based on the present work and combined with results in our previous publications [4-8,20,47,48].
Funding
This research was funded in part by the National Science Foundation (EPSCoR Cooperative Agreements OIA-1655280 and OIA-2148653; HBCU-UP Grant Number 2107542) and in part by the Army Research Office (Grant Numbers W911NF-18-1-0446 and W911NF1910506). Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation and the Army Research Office.
Author contributions
A.G.V. conceived the idea, analyzed the data, and participated in manuscript writing. All authors provided the data and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
- Dubinov AE, Lazarenko ER, Selemir VD. Effect of glow discharge air plasma on grain crops seed. IEEE Trans Plasma Sci. 2000; 28:180-183. doi 10.1109/27.842898.
- Filipov AK, Fedorov MA, Filipov DA. Method of pre-sowing treatment of plant seeds. Russian patent RU2293456C1, 2007.
- Yan D, Lin L, Zvansky M, Kohanzadeh L, Taban S, Chriqui S, Keidar M. Improving Seed Germination by cold atmospheric plasma. Plasma. 2022; 5:98-110. https://doi.org/10.3390/plasma5010008.
- Volkov AG, Hairston JS, Marshal J, Bookal A, Dholichand A, Patel D. Plasma seeds: Cold plasma accelerates Phaseolus vulgaris seeds' imbibition, germination, and speed of seedling growth. Plasma Med. 2020; 10:139–158. https:/doi.org/10.1615/PlasmaMed.2020036438.
- Volkov AG, Hairston JS, Patel D, Gott RK, Xu KG. Cold plasma poration and corrugation of pumpkin seed coats. Bioelectrochem. 2019; 128:175-185. doi:10.1016/j.bioelechem.2019.04.012.
- Volkov AG, Xu KG, Kolobov VI. Cold plasma interactions with plants: Morphing and movements of Venus flytrap and Mimosa pudica induced by argon plasma jet. Bioelectrochemistry. 2017 Dec;118:100-105. doi: 10.1016/j.bioelechem.2017.07.011. Epub 2017 Jul 29. PMID: 28780442.
- Volkov AG, Xu KG, Kolobov VI. Plasma-generated reactive oxygen and nitrogen species can lead to closure, locking and constriction of the Dionaea muscipula Ellis trap. J R Soc Interface. 2019 Jan 31;16(150):20180713. doi: 10.1098/rsif.2018.0713. PMID: 30958146; PMCID: PMC6364641.
- Volkov AG. Cold atmospheric pressure He-plasma jet and plasma ball interactions with the Venus flytrap: Electrophysiology and side effects. Bioelectrochemistry. 2021 Aug;140:107833. doi: 10.1016/j.bioelechem.2021.107833. Epub 2021 May 1. PMID: 33989989.
- Nakano R, Tashiro K, Aijima R, Hayashi N. Effect of oxygen plasma irradiation on gene expression in plant seeds induced by active oxygen species. Plasma Med. 2016; 6:303-13.
- Pua N, Zivkovi S, Selakovi N, Milutinovi M, Boljevi J, Malovi G, Petrovi Z. Long and short-term effects of plasma treatment on meristematic plant cells. Appl Phys Lett. 2014; 104:214106. doi 10.1063/1.4880360.
- Kobayashi M, Wang Y, Kumagai S, Uraoka Y , Ito T. Effects of cold atmospheric plasma irradiation on Arabidopsis seedlings. Japanese J Appl Physics. 2020; 59: SAAB09. doi:10.7567/1347-4065/ab4e7b.
- Lazović S, Maletić D, Leskovac A, Filipović J, Puač N, Malović G, Joksić G, Petrović Z. Plasma induced DNA damage: comparison with the effects of ionizing radiation. Appl Phys Lett. 2014; 105:124101. doi: 10.1063/1.4896626
- Adhikari B, Adhikari M, Ghimire B, Adhikari BC, Park G, Choi EH. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic Biol Med. 2020 Aug 20;156:57-69. doi: 10.1016/j.freeradbiomed.2020.06.003. Epub 2020 Jun 17. PMID: 32561321.
- Tomeková J , Kyzek S , Medvecká V , Gálová E , Zahoranová A. Influence of cold atmospheric pressure plasma on pea seeds: DNA damage of seedlings and optical diagnostics of plasma. Plasma Chem. Plasma Proces. 2020; 40:1571–1584.
- Marzban M, Farahani F, Atyabi SM, Noormohammadi Z. Induced genetic and chemical changes in medicinally important plant Catharanthus roseus (L.) G. Don: cold plasma and phytohormones. Mol Biol Rep. 2022 Jan;49(1):31-38. doi: 10.1007/s11033-021-06789-w. Epub 2021 Nov 13. PMID: 34773551.
- Seol YB, Kim J, Park SH, Young Chang H. Atmospheric Pressure Pulsed Plasma Induces Cell Death in Photosynthetic Organs via Intracellularly Generated ROS. Sci Rep. 2017 Apr 3;7(1):589. doi: 10.1038/s41598-017-00480-6. PMID: 28373681; PMCID: PMC5428426.
- Švubová R , Kyzek S , Medvecká V , Slováková I , Gálová E, Zahoranová A. Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. cv. Prophet) Seeds. Plasma Chem. Plasma Processing. 2020; 40:1221–40.
- Tesla N. Incandescent electric light. U.S. Patent Number 0.514.170, 1894.
- Burin M J, Simons G G , Ceja H G, Zweben S J , Nagy A , Brunkhorst C. On filament structure and propagation within a commercial plasma globe. Phys Plasmas. 2015; 22:053509. doi:10.1063/1.4919939.
- Volkov AG, Bookal A, Hairston JS, Patel D. Radio frequency plasma capacitor can increase rates of seeds imbibition, germination, and radicle growth. Funct Plant Biol. 2021 Feb;48(3):312-320. doi: 10.1071/FP20293. PMID: 33220717.
- Ark A , Parry W. Application of high-frequency electrostatic fields in agriculture. The Quaternary Review Biol. 1940; 15:172-91.
- Bertholon MDE. The electricity of plants: Book in which we deal with the electricity of the atmosphere on plants, its effects on the economy of plants, their medical virtues. (P.F. Didotjeune, Paris, France) 1783.
- Bois-Reymond DUE. Investigations on animal electricity. 1: 7-10. (Published by G. Reimer, Berlin, Germany) 1848.
- Bose J C. Transmission of Stimuli in Plants. Nature. 1925; 115:457-7.
- Ksenzhek O S ; Volkov A G. Plant Energetics. (Academic Press, San Diego, US) 1998.
- Volkov A G. Plant Electrophysiology. Methods and Cell Electrophysiology. (Springer, Berlin, Germany) 2012.
- Volkov A G. Plant Electrophysiology. Signaling and Responses. (Springer, Berlin, Germany) 2012.
- Solly E. On the influence of electricity on vegetation. J Horticultural Soc London. 1846; 1:81-109.
- Murr L E. Plant growth response in a simulated electric field environment. Nature. 1963; 200:490–491. doi 10.1038/200490b0.
- Lemström K. Electricity in Agriculture and Horticulture. (Electrician Publications, London, UK, 1904.
- Volkov AG, Foster JC, Jovanov E, Markin VS. Anisotropy and nonlinear properties of electrochemical circuits in leaves of Aloe vera L. Bioelectrochemistry. 2011 Apr;81(1):4-9. doi: 10.1016/j.bioelechem.2010.11.001. Epub 2010 Nov 27. PMID: 21167797.
- Volkov A G , O’Neal L , Volkova-Gugeshashvili M I , Markin V S. Electrostimulation of Aloe Vera L., Mimosa Pudica L. and Arabidopsis Thaliana: Propagation and collision of electronic potentials. J Electrochem Soc. 2013; 160:G3102-G3111.
- Volkov AG, Shtessel YB. Electrical signal propagation within and between tomato plants. Bioelectrochemistry. 2018 Dec;124:195-205. doi: 10.1016/j.bioelechem.2018.08.001. Epub 2018 Aug 7. PMID: 30125795.
- Jack J J , Noble D , Tsien R W. Electric Current Flow in Excitable Cells. Clarendon, Oxford, 1975.
- Hodgkin AL, Rushton WA. The electrical constants of a crustacean nerve fibre. Proc R Soc Med. 1946 Dec 3;134(873):444-79. doi: 10.1098/rspb.1946.0024. PMID: 20281590.
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969; 58:631-639.
- Kyzek S, Holubova L , Medvecká V , Tomekova J, Gálová E , Zahoranová A. Cold atmospheric pressure plasma can induce an adaptive response in pea seeds. Plasma Chem. Plasma Processing. 2019; doi: 10.1007s11090-018-9951-x.
- Stolarik T , Henselova M , Martinka M, Novak O , Zahoranova A , Cernak M. Effect of low-temperature plasma on the structure of seeds, growth, and metabolism of exogenous phytohormones in pea (Pisum sativum L.) Plasma Chemistry and Plasma Processing. 2015. doi: 10.1007/s11090-015-9627-8
- Lackmann JW, Schneider S, Edengeiser E, Jarzina F, Brinckmann S, Steinborn E, Havenith M, Benedikt J, Bandow JE. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J R Soc Interface. 2013 Sep 25;10(89):20130591. doi: 10.1098/rsif.2013.0591. PMID: 24068175; PMCID: PMC3808546.
- Lv X, Cheng JH. Evaluation of the Effects of Cold Plasma on Cell Membrane Lipids and Oxidative Injury of Salmonella typhimurium. Molecules. 2022 Jan 19;27(3):640. doi: 10.3390/molecules27030640. PMID: 35163904; PMCID: PMC8838372.
- Attri P, Kumar N, Park JH, Yadav DK, Choi S, Uhm HS, Kim IT, Choi EH, Lee W. Influence of reactive species on the modification of biomolecules generated from the soft plasma. Sci Rep. 2015 Feb 4;5:8221. doi: 10.1038/srep08221. PMID: 25649786; PMCID: PMC4316168.
- Takai E , Kitamura T , Kuwabara J , Ikawa S , Yoshizawa S , Shiraki K , Kawasaki H , Arakawa R , Kitano K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J Phys D Appl Phys. 2014; 47:285403. https://doi.org/10.1088/0022-3727/47/28/285403.
- Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006 Jun;141(2):384-90. doi: 10.1104/pp.106.078295. PMID: 16760492; PMCID: PMC1475453.
- Lu X , Naidis G V , Laroussi M , Reuter S , Graves D B , Ostrikov K. Reactive species in non-equilibrium atmospheric pressure plasmas: Generation, transport, and biological effects. Phys Rep. 2016; 630:1–84.
- Hill A C , Pack M R , Treshow M , Downs R J , Transtrum L G. Plant injury induced by ozone. Phytopathology. 1961; 51:356-63.
- Patton R L , Garraway M O. Ozone-induced necrosis and increased peroxidase activity in hybrid poplar leaves. Environm. Experimental Bot. 1986; 26:137-141.
- Volkov A G , Bookal A , Hairston J S , Roberts J, Taengwa G, Patel D. Mechanisms of multielectron reactions at the plasma/water interface: Interfacial catalysis, RONS, nitrogen fixation, and plasma-activated water. Electrochim Acta. 2021; 385:138441. doi:10.1016/j.electacta.2021.138441.
- Volkov AG, Hairston JS, Taengwa G, Roberts J, Liburd L, Patel D. Redox Reactions of Biologically Active Molecules upon Cold Atmospheric Pressure Plasma Treatment of Aqueous Solutions. Molecules. 2022 Oct 19;27(20):7051. doi: 10.3390/molecules27207051. PMID: 36296644; PMCID: PMC9608965