More Information
Submitted: March 14, 2024 | Approved: April 30, 2024 | Published: May 01, 2024
How to cite this article: Edewor TI, Olasunkanmi AM, Owa SO. GS-MS Profile, Total Flavonoid and Phenolic Contents and Antioxidant Capacity of Leaves of Vitelleria paradoxa c.f. Gaertn. J Plant Sci Phytopathol. 2024; 8: 043-047.
DOI: 10.29328/journal.jpsp.1001131
Copyright License: © 2024 Edewor TI, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: GC-MS; Flavonoid; Phenolic; Antioxidant; Leaves; Vitelleria paradoxa
GS-MS Profile, Total Flavonoid and Phenolic Contents and Antioxidant Capacity of Leaves of Vitelleria paradoxa c.f. Gaertn
Theresa Ibibia Edewor1*, Amuda Mutiu Olasunkanmi1 and Stephen Oluwagbemiga Owa2
1Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria
2Department of Biological Sciences, Landmark University, Omu Aran, Kwara State, Nigeria
*Address for Correspondence: Theresa Ibibia Edewor, Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria, Email: [email protected]
Vitellaria paradoxa is an important medicinal plant that is used for the treatment of infections such as diarrhea, dysentery, helminthes, gastrointestinal tract, skin, and wounds. This research aims to determine other important uses of the plant leaves and quantify the phytochemicals present in the leaves. The plant leaves were extracted with two solvents (n-hexane and methanol). The phytochemicals were qualitatively and quantitatively analyzed using standard methods. The antioxidant activity was determined using DPPH. In the qualitative phytochemical screening of the methanol extract flavonoids, alkaloids, saponins, and tannins were identified as being present while steroids, anthraquinones, and glycosides were absent. All the screened secondary metabolites were absent in the n-hexane extract. In the GC-MS analysis of the methanol and n-hexane extracts seven compounds were obtained from the methanol extract while a total of twenty-four compounds were obtained from the n-hexane extract. The quantitative determination of the total flavonoid and phenolic contents showed that the leaves high content of flavonoids (91.00 mg quercetin equivalent/g extract) and phenolics (91.39 mg Gallic acid equivalent /g extract). These phytochemicals could be responsible for its high antioxidant activity (79.62%).
Medicinal plants are sources of medicine for both traditional and modern medicine. Plants used in traditional medicine have not been fully studied while about 25% of the prescribed drugs in the industrialized nations are derived from medicinal plants [1]. The advent of civilization and modernization is taking a toll on the use of medicinal plants as herbal drugs [2] in that the younger generation is not interested in traditional health practices. However many countries are now involved in the screening of plants for their medicinal purposes. Researchers have shown that plants contain phytochemicals that are used either directly or indirectly as precursors or lead compounds in pharmaceutical industries and as such are considered as the bedrock for modern medicine [3]. Scientific research into medicinal plants has resulted in the isolation of bioactive compounds such as morphine, etc; therefore plant secondary metabolites are considered potential sources of new drugs, antibiotics, insecticides, and herbicides [4]. Some medicinal plants possess natural antioxidants which have been proven to be effective in preventing the destructive processes caused by oxidative stress. Interest in naturally occurring antioxidants has increased in the food, cosmetic, and pharmaceutical industries because they possess a diverse multitude and magnitude of activity and provide enormous scope in correcting the imbalance. These antioxidants can scavenge free radicals [5] Govind, 2011 [6] proposed that the use of medicinal plants with high levels of antioxidant constituents will be effective in the treatment of hepatic damage.
Vitellaria paradoxa is a member of the Sapotaceae family. And it is indigenous to Africa [7]. Different parts of the plant are used for the treatment of infections such as diarrhea, dysentery, helminthes, gastrointestinal tract, skin, and wound [8]; the stem bark is used to suppress cough and also for the treatment of leprosy [9]. Most research on this plant is on the seed which is rich in oil, therefore this research aims to evaluate the importance of the leaf by determining the total phenolic and flavonoid (91.00 quercetin equivalent/g extract) contents and the phytochemical profile of the leaf extract.
General
The chemicals used for this analysis were methanol, n-hexane, concentrated sulfuric acid, iron (III) chloride, Fehling solution, Meyer’s reagent, sodium hydroxide, sodium carbonate, Folin-Ciocalteau reagent, Aluminum chloride, Sodium nitrite which were obtained from E. Merck (Darmstadt, Germany) while 1, 1-diphenyl-2-picrylhydrazyl (DPPH), Gallic acid and quercetin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Absorbance measurements were recorded on a Genseys 10S vI. 200 217H311008 spectrophotometer.
Sample preparation and extraction
The plant samples were collected during the rainy season from a traditional health practitioner (Someone who uses herbs for treating ailments) in Omu Aran, Kwara State, Nigeria. Omu Aran has the geographical coordinates, latitude 8.1402266 and longitude 5.0963141. The plant sample was taken to the Department of Pure and Applied Biology for proper identification. A voucher number LHO 592 was assigned to the sample. The leaves were air-dried under laboratory conditions for two weeks, pulverized into fine powder, and stored in an air-tight container until when required for use. The leaves were extracted with two different solvents (n-hexane and methanol) using a Soxhlet extractor. The extracts were concentrated using a rotary evaporator. Later, it was evaporated to dryness. The extracts obtained were subjected to the required analysis.
Phytochemical studies
Phytochemical screening: The extracts were screened for the presence of alkaloids, flavonoids, steroids, saponins, glycosides, anthraquinones, and tannins using the method described by Harborne, 1999.
GC-MS analysis: The GC (7890A) – MS (5975C) used for the analysis was from Agilent, USA. It has a triple-axis detector that is equipped with an auto-injector. The carrier gas was helium. The capillary column used for the separation was 30 m in length and had an internal diameter of 0.2 µm and thickness of 250 µm. The ion source temperature was 250 °C and the interface temperature of 300 °C. Other operation conditions were the pressure of 16.2 psi, injector size of 1 µL which is in split mode with a split ratio of 1:5, and injection temperature of 300 °C. The column temperature was started at 35 °C for 5 minutes and later increased to 150 °C at the rate of 4 °C/min. This temperature was further increased to 250 C at the rate of 20 °C/min for 5 mins. It took 45.5 mins to complete elution. The compounds separated by the GC were identified by comparing their MS data with those of standard MS from NIST, 2011 library incorporated into the operating system.
Determination of total flavonoid content of the methanolic extract
A dilute solution of the extract was prepared by adding 5 ml of methanol to 5 mg of the extract to obtain a concentration of 1 mg/ml. To 0.3 ml of this solution was added 3.4 ml of 30% methanol, 0.15 ml of NaNO2 (0.5M), and 0.15 ml of Al Cl3.6H2O (0.3M) and mixed for 5 mins using a vortex mixer. Later 1 ml of NaOH (1M) was added to the mixture and stirred properly. The absorbance of the mixture was measured at 506 against a reagent blank. Quercetin was used as the standard. The standard curve for obtaining the total flavonoid content was derived by preparing serial dilutions of stock solution of quercetin and their absorbance was measured at 506 nm. The total flavonoid content is expressed as mg of quercetin equivalent/g extract.
Determination of total phenolic content of the methanolic extract
5 mg of the extract was dissolved in 5 ml of distilled water to obtain a concentration of 1mg/ml. 0.5 ml of Folin-Ciocalteau reagent was added to it. After 5 mins 20 ml of sodium carbonate and 26 ml of distilled water were added to the mixture and stirred properly. The mixture was left standing in the dark for 2 hours at room temperature. Then the absorbance was measured at 765 nm.
Evaluation of the antioxidant activity of the methanolic extract
The antioxidant capacity of the methanolic leaf extract was determined using the DPPH (2, 2-dipheyl-1-picrylhydrazyl) radical. The DPPH (4 mg) was dissolved in methanol (100ml) and different concentrations of the extract were prepared (50-200 µg/ml). 2 ml each was taken from the DPPH solution and introduced into each of the extract dilutions and left in the dark for 20 mins. 2 ml of DPPH in methanol was used as the negative control while ascorbic acid was used as the positive control. The following equation was used to determine the DPPH radical scavenging capacity of the leaf extract:
Where As = absorbance of the sample
Ab = absorbance of blank
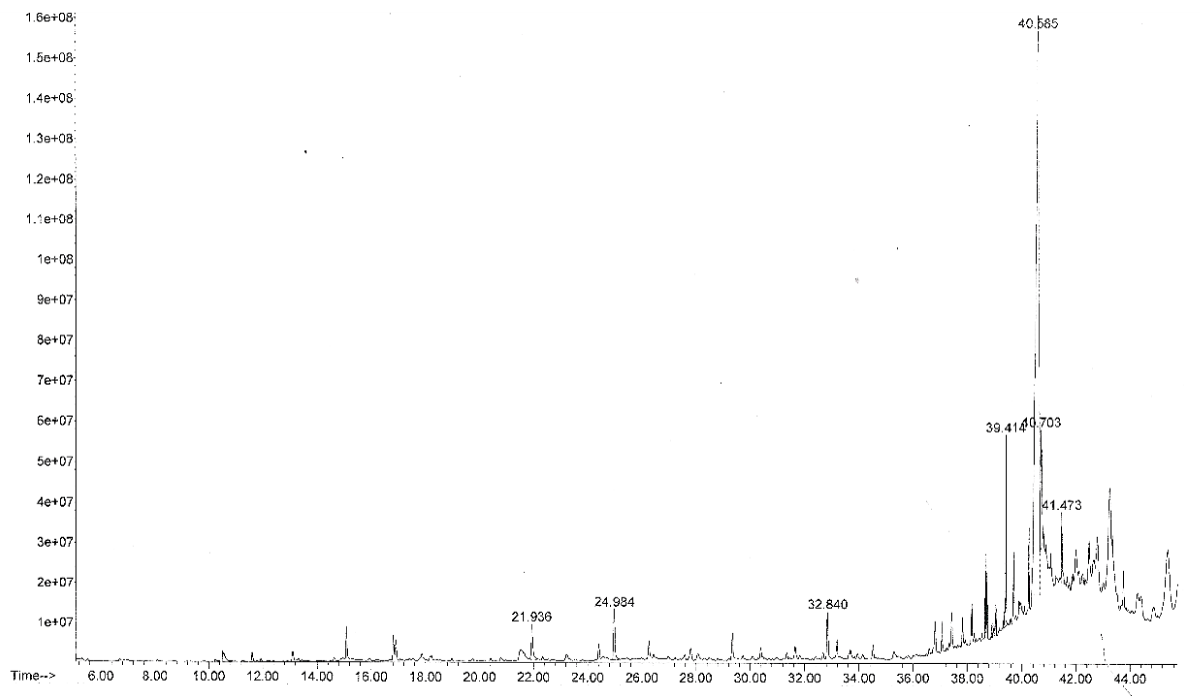
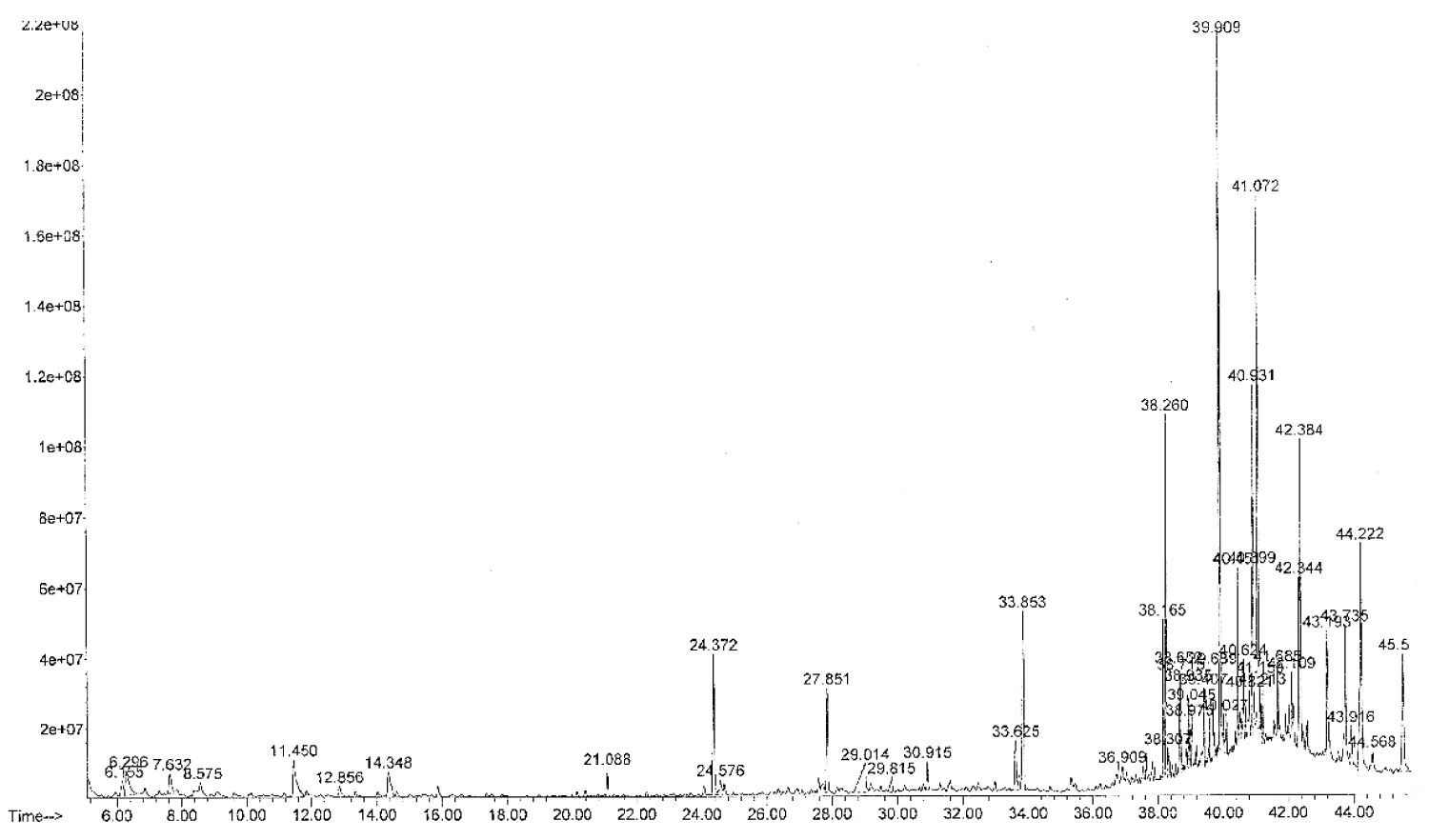
Before taking steps to isolate individual bioactive compounds present in any medicinal plant, it is necessary to explore the importance of determining the type of secondary metabolites present as it gives a broad idea regarding the nature of the phytochemicals in the medicinal plant. In this study, the phytochemical screening of the methanol extract showed the presence of the following types of secondary metabolites - flavonoids, alkaloids, saponins, and tannins while steroids, anthraquinones, and glycosides were absent. All the screened secondary metabolites were absent in the n-hexane extract. The result is presented in Table 1. The GC-MS analysis of the methanol extract separated seven compounds as shown in Figure 1 while 4 were identified as shown in Table 2. The identified compounds belong to the class of compounds known as furan, phenolics, and fatty acid esters. Compound 5 has the highest percent composition (78.57%) while compound 1 has the lowest (2.57%). In the GC-MS analysis of the n-hexane extract, a total of twenty-four compounds were separated (Figure 2) and some were identified. The first compound to emerge was thymol with a retention time of 24.37 mins and a percentage composition of 3.61% while the last to emerge was heptacosane with a retention time of 45.51 mins and a percentage composition of 3.34% as presented in Table 3. Compound 12 has the highest composition of 14.60%. Others with appreciable composition are compounds 18 (9.94%) and octacosane (6.92%).
| Table 1: Phytochemical screening of leaf extracts of Vitellaria paradoxa. | |||||||
| Flav | ster | sap | Alk | Tan | Anthr | Gly | |
| Met | + | - | + | + | + | - | - |
| n-hex | - | - | - | - | - | - | - |
| Key: Flav: Flavonoids; Ster: Steroids; Sap: Saponins; Alk: Alkaloids; Tan: Tannins; Anthr: Anthraquinones; Glycosides: - - negative; + - positive | |||||||
| Table 2: GC-MS report on the methanol extract. | |||||
| Peak | Retention time (min) | % of composition | Name of compound | Molecular weight (g/mol) | Molecular formula |
| 1 | 21.936 | 2.57 | Benzofuran, 2,3-dihydro | 120 | C8H8O |
| 2 | 24.984 | 2.95 | 2-methoxy-4-vinylphenol | 150 | C9H10O2 |
| 3 | 32.840 | 2.64 | - | - | - |
| 4 | 39.414 | 4.47 | Hexadecanoic acid, methyl ester | 150 | C17H34O2 |
| 5 | 40.585 | 78.57 | Not identified | - | - |
| 6 | 40.703 | 6.00 | Not identified | - | - |
| 7 | 41.473 | 2.8 | Not identified | - | - |
| Table 3: GC-MS report on the n-hexane extract. | |||||
| Peak number | Retention time (min) | % composition | Name of compound | Molecular weight (g/mol) | Molecular formula |
| 1 | 24.372 | 3.607 | Thymol | 150 | C10H14O |
| 2 | 27.851 | 2.417 | Tetradecane | 198 | C14H30 |
| 3 | 33.625 | 1.283 | Cetene | 224 | C16H32 |
| 4 | 33.853 | 4.814 | Hexadecane | 226 | C16H34 |
| 5 | 38.165 | 2.288 | 1-octadecene | 252 | C18H36 |
| 6 | 38.260 | 4.842 | Not identified | - | - |
| 7 | 38.652 | 1.145 | Not identified | - | - |
| 8 | 38.715 | 1.073 | 2-pentadecanone, 6,10,14-trimethyl | 268 | C18H36O |
| 9 | 38.935 | 0.854 | 1,2-benzene dicarboxylic acid, Bis(2-methyl propyl)ester | 276 | C16H20O4 |
| 10 | 39.045 | 0.879 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 296 | C20H40O |
| 11 | 39.689 | 1.096 | 1,2-benzene dicarboxylic acid, butyl-8-methylnonyl ester | 362 | C22H34O4 |
| 12 | 39.909 | 14.599 | Not identified | - | - |
| 13 | 40.027 | 0.447 | Not identified | - | - |
| 14 | 40.452 | 1.732 | Not identified | - | - |
| 15 | 40.624 | 1.083 | Phytol | 296 | C20H40O |
| 16 | 40.899 | 1.716 | Linoleic acid ethyl ester | 308 | C20H36O2 |
| 17 | 40.931 | 3.341 | Ethyl oleate | 310 | C20H38O2 |
| 18 | 41.072 | 9.942 | Not identified | - | - |
| 19 | 42.344 | 2.240 | Not identified | - | - |
| 20 | 42.384 | 4.375 | Tetracosane | 338 | C24H50 |
| 21 | 43.193 | 2.955 | Heptacosane | 380 | C27H56 |
| 22 | 43.735 | 2.907 | Diisooctylphthalate | 400 | C25H36O4 |
| 23 | 44.222 | 6.918 | Octacosane | 394 | C28H58 |
| 24 | 45.510 | 3.335 | Heptacosane | 380 | C27H56 |
Table 4 shows the total flavonoid and phenolic contents of the methanol extract. The values obtained indicate that the methanol extract is rich in flavonoids and phenolics. The high antioxidant activity of the methanol extract as indicated in Table 4 could be a result of the abundance of flavonoids and phenolics in it. The configuration, number of hydroxyl groups, structural class, degree of polymerization, and substituents on the nuclear structure determine its bioactivity, and metabolism of flavonoids [10]. Epidemiological studies have shown that flavonoids possess versatile human health benefits such as antioxidant, anti-inflammatory, anticancer, antiviral, antimicrobial, etc. Due to the presence of hydroxyl groups in flavonoids, they can act as antioxidants by scavenging free radicals and /or by chelating metal ions. This action can prevent the generation of free radicals which can damage target molecules [11,12]. In plants, flavonoids can act as secondary antioxidant defense systems in tissues that are exposed to different abiotic and biotic stresses, and also as growth regulators. In foods, they are responsible for the colour, stability, flavour, prevention of oxidation, and protection the enzymes and vitamins [12-14]. In recent years, researchers and industries have shown increasing interest in phenolics due to their health benefits. Researchers have reported that the intake of phenolics is related to a lower incidence of chronic degenerative diseases such as cancer, diabetes, etc. Phenolics can enhance glutathione S-transferases, reduce the formation of hydrogen peroxide, prevent the damage of DNA, inhibit intestinal glucose absorption, inhibit adipogenesis, and modulate reactive oxygen species levels in the intestinal contents [16,17].
Table 4: Total flavonoid, phenolic contents, and antioxidant activity of the methanolic extract. |
|||
| Parameters | Total flavonoid content(expressed in quercetin equivalent/g extract) | Total phenolic content(expressed in Gallic acid equivalent /g extract) | DPPH Scavenging activity (%) |
| Values | 91.00 | 91.39 | 79.62 |
The screened secondary metabolites- flavonoids, alkaloids, saponins, and tannins were present in the methanol extract while steroids, anthraquinones, and glycosides were absent. All the screened metabolites were absent in the n-hexane extract. The GC-MS analysis of the methanol extract showed that the compounds present were furans, phenolics, and fatty acid esters. The total phenolic and flavonoid contents were high. This could be responsible for the high antioxidant activity of the plant leaves. Further work is ongoing to determine the exact components in the plant leaves that are responsible for the antioxidant activity of the plant leaves.
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000 Jun;17(3):215-34. doi: 10.1039/a902202c. PMID: 10888010.
- Cox PA. The Seven Pillars of Ethnomedical Wisdom. Ethnobotany. 2005; 17:24-34.
- Evans CE, Banso A, Samuel OA. Efficacy of some nupe medicinal plants against Salmonella typhi: an in vitro study. J Ethnopharmacol. 2002 Apr;80(1):21-4. doi: 10.1016/s0378-8741(01)00378-6. PMID: 11891083.
- Crozier A, Clifford MN, Ashihara H. Plant Secondary metabolites: Occurrence, structure, and role in the human diet. Wiley-Blackwell. 2006. ISBN: 978-18405-12509-3.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012 Nov 16;12:221. doi: 10.1186/1472-6882-12-221. PMID: 23153304; PMCID: PMC3524761.
- Govind P. Medicinal plants against liver diseases IJPR. 2011; 2:115- 121.
- Byakagaba P. Population structure and regeneration status of Vitellaria paradoxa (C.F) Gaertn under different land management regimes in Uganda. Agricultural Journal. 2011; 6(1):14-22.
- Soladoye MO, Orhiere SS, Ibimode BM. Ethnobotanical study of two indigenous multipurpose plants in the Guinea savanna of Kwara State – Vitellaria paradoxa and Parkia biglobosa. Biennial conference of Ecological Society of Nigeria. Forest Research Institute, Ibadan. 1989; 13.
- Ferry MP, Gessain M, Geeain R. Vegetative propagation of Shea, Kola, and Pentadesma. Cocoa Research Institute, Ghana annual Report (1987/88). 1974; 98-100.
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002 Oct;13(10):572-584. doi: 10.1016/s0955-2863(02)00208-5. PMID: 12550068.
- Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem. 2006 Aug 23;54(17):6343-51. doi: 10.1021/jf060986h. PMID: 16910729.
- Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement Altern Med. 2013 May 30;13:120. doi: 10.1186/1472-6882-13-120. PMID: 23721571; PMCID: PMC3680177.
- Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999 Sep;70(3 Suppl):491S-499S. doi: 10.1093/ajcn/70.3.491s. PMID: 10479221.
- Kumar S, Sharma UK, Sharma AK, Pandey AK. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cell Mol Biol (Noisy-le-grand). 2012 Dec 22;58(1):174-81. PMID: 23273209.
- Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004 Summer;59(3):113-22. doi: 10.1007/s11130-004-0049-7. PMID: 15678717.
- Miene C, Klenow S, Veeriah S, Richling E, Glei M. Impact of apple polyphenols on GSTT2 gene expression, subsequent protection of DNA and modulation of proliferation using LT97 human colon adenoma cells. Mol Nutr Food Res. 2009 Oct;53(10):1254-62. doi: 10.1002/mnfr.200800444. PMID: 19753602.
- Serra AT, Rocha J, Sepodes B, Matias AA, Feliciano RP, de Carvalho A, Bronze MR, Duarte CM, Figueira ME. Evaluation of cardiovascular protective effect of different apple varieties - Correlation of response with composition. Food Chem. 2012 Dec 15;135(4):2378-86. doi: 10.1016/j.foodchem.2012.07.067. Epub 2012 Jul 20. PMID: 22980816.