More Information
Submitted: March 04, 2024 | Approved: March 18, 2024 | Published: March 19, 2024
How to cite this article: Tsygankova VA, Andrusevich YaV, Vasylenko NM, Kopich VM, Popilnichenko SV, et al. Auxin-like and Cytokinin-like Effects of New Synthetic Pyrimidine Derivatives on the Growth and Photosynthesis of Wheat. J Plant Sci Phytopathol. 2024; 8: 015-024.
DOI: 10.29328/journal.jpsp.1001126
Copyright License: © 2024 Tsygankova VA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Triticum aestivum L.; Auxin IAA; Plant growth regulators; Methyur; Kamethur; Pyrimidine derivatives
Auxin-like and Cytokinin-like Effects of New Synthetic Pyrimidine Derivatives on the Growth and Photosynthesis of Wheat
Tsygankova Victoria Anatolyivna* , Andrusevich YaV, Vasylenko NM, Kopich VM, Popilnichenko SV, Pilyo SG and Brovarets VS
, Andrusevich YaV, Vasylenko NM, Kopich VM, Popilnichenko SV, Pilyo SG and Brovarets VS
Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of Sciences of Ukraine, 1, Academician Kukhar str., 02094, Kyiv-94, Ukraine
*Address for Correspondence: Dr. Tsygankova Victoria Anatolyivna, Biol. Sci., Principal researcher, Senior Staff Scientist, Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry, National Academy of Sciences of Ukraine, 1, Academician Kukhar str., 02094, Kyiv-94, Ukraine, Email: [email protected]
The regulatory effect of new synthetic thienopyrimidine derivatives on the growth and photosynthesis of wheat (Triticum aestivum L.) variety Svitlana in the vegetative phase was studied. The regulatory effect of new synthetic thienopyrimidine derivatives was compared with the regulatory effect of auxin IAA (1H-indol-3-yl)acetic acid) or synthetic plant growth regulators Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine) and Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine). After 2 weeks, morphometric parameters (such as average length of shoots and roots (mm), average biomass of 10 plants (g)) and biochemical parameters (such as content of photosynthetic pigments (µg/ml)) of wheat plants grown from seeds treated with synthetic thienopyrimidine derivatives, or auxin IAA, or synthetic plant growth regulators Methyur and Kamethur at a concentration of 10-6M, were measured and compared with similar parameters of control wheat plants grown from seeds treated with distilled water. The regulatory effect of new synthetic thienopyrimidine derivatives on the morphometric and biochemical parameters of wheat plants was similar or higher compared to the regulatory effect of auxin IAA, or synthetic plant growth regulators Methyur and Kamethur. The relationship between the chemical structure of new synthetic thienopyrimidine derivatives and their regulatory effect on the growth and photosynthesis of wheat plants was revealed. The most biologically active thienopyrimidine derivatives are proposed to be used as new synthetic physiological analogues of auxins and cytokinins to improve growth and increase photosynthesis of wheat (Triticum aestivum L.) variety Svitlana in the vegetative phase.
As is known, phytohormones auxins and cytokinins are key regulators of plant growth and development, which are synthesized in the apical meristems of shoots and roots, young leaves, seeds, and fruits [1-4]. They exhibit a stimulating effect on seed germination, the formation and growth of shoots, and adventitious and lateral roots of plants in the vegetative stage [1-4]. Considerable attention of plant biologists is devoted to the screening of new effective analogues of auxins and cytokinins of synthetic origin for their use in agriculture to improve growth and increase the productivity of agricultural crops. In recent years, new synthetic analogues of auxins and cytokinins have been created, such as NAA (1-naphthylacetic acid), 2,4-D (2,4-dichlorophenoxyacetic acid), 3,4-D (3,4-dichlorophenoxyacetic acid), 2,4,5-T (2,4,5-trichlorophenoxyacetic acid), 4-CPA (4-chloro-pheno xyacetic acid), dicamba (3,6-dichloro-2-methoxybenzoic acid), picloram (4-amino-3,5,6-trichloropyridine-2-carboxylic acid), kinetin (6-furfurylaminopurine), 2iP (N6-(2-isopentenyl) adenine), BA (N6-benzyladenine), BAP (6-benzylaminopurine), BPA (N-benzyl-9-(2-tetrahydropyranyl)-adenine), tetrahydro pyranyl-benzyladenine (PBA), TDS (thidiazuron), that have a physiological effect similar to natural phytohormones such as IAA (indole-3-acetic acid), 4-Cl-IAA (4-chloro-IAA), PAA (phenylacetic acid), IBA (indole-3-butyric acid), IPA (indole-3-pyruvic acid), 2-(2,4-dichloro-phenoxy) propionic acid (2,4-DP), indole-3-lactic acid (ILA), zeatin (N6-(4-Hydroxy-3-methyl-2-buten-1-yl)adenine) on the growth and development of plants during ontogenesis, due to which they are used in agriculture as plant growth regulators [3-13].
Our previously conducted studies show that the synthetic plant growth regulators Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), and new synthetic pyrimidine derivatives when used in concentrations from 10-5M to 10-8M, demonstrate auxin-like and cytokinin-like effects on growth and development, as well as on the productivity of major grain, leguminous, vegetable, industrial and horticultural crops [14-22].
Currently, new synthetic compounds belonging to thienopyrimidine derivatives are used in medicine as therapeutic agents, showing antibacterial, antifungal, antiviral, anticancer, antioxidant, anti-inflammatory, antitubercular, antidiabetic, antihypertensive, cardiotonic, anticonvulsant, antimalarial, antihelminthic and analgesic activities through inhibition of various enzymes and pathways [23-30]. Besides this, a very promising approach is the screening of new synthetic compounds among thienopyrimidine derivatives that can be practically used in agriculture as plant growth regulators, herbicides, pesticides, and insecticides by inhibiting various enzymes of weeds and insects [31-39].
Our present work is aimed at the screening of new synthetic compounds among thienopyrimidine derivatives, which show the ability to demonstrate auxin-like and cytokinin-like effects on the growth and photosynthesis of an important agricultural crop - wheat (Triticum aestivum L.) variety Svitlana.
Synthetic plant growth regulators Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) were synthesized at the Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine. Auxin IAA (1H-indol-3-yl)acetic acid was manufactured by Sigma-Aldrich, USA (Table 1).
Table 1: Chemical structure, name, and relative molecular weight of auxin IAA, synthetic plant growth regulators Methyur (sodium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), Kamethur (potassium salt of 6-methyl-2-mercapto-4-hydroxypyrimidine), and new synthetic thienopyrimidine derivatives (compounds № 1 – 11).
Plant growth conditions
The seeds of wheat (Triticum aestivum L.) variety Svitlana were sterilized with 1% KMnO4 solution for 15 min, then treated with 96% ethanol solution for 1 min, after which they were washed three times with sterile distilled water. After this procedure, wheat seeds were placed in the plastic cuvettes (each containing 20 seeds - 25 seeds) on the perlite moistened with distilled water (control sample) or water solutions of auxin IAA, or synthetic plant growth regulators Methyur and Kamethur, or synthetic thienopyrimidine derivatives used in the most physiologically active concentration of 10-6M (experimental samples). Then the wheat seeds were placed in a thermostat for germination in the dark at a temperature of 20 °C - 22 °C for 48 hours. After the appearance of wheat seedlings, they were placed in a climate chamber, where they were grown for 2 weeks at 16/8 h light/dark conditions, at the temperature of 20 °C - 22 °C, light intensity of 3000 lux, and air humidity of 60% - 80%. Comparative analysis of morphometric parameters of wheat plants (average length of shoots and roots (mm), average biomass of 10 plants (g)) was carried out at the end of the two-week period according to the method [40].
Determination of the content of photosynthetic pigments
To perform the extraction of photosynthetic pigments, we homogenized a sample (500 mg) of wheat leaves in the porcelain mortar cooled at the temperature of 10 °С 96% ethanol at the ratio of 1: 10 (weight: volume) with the addition of 0,1 - 0,2 g CaCO3 (to neutralize the plant acids). The 1 ml of obtained homogenate was centrifuged at 8000 g in a refrigerated centrifuge K24D (MLW, Engelsdorf, Germany) for 5 min at the temperature of 4 °С. The obtained precipitate was washed three times, with 1 ml 96% ethanol, and centrifuged at the above-mentioned conditions. After this procedure, the optical density of chlorophyll a, chlorophyll b, and carotenoid in the obtained extract was measured using a spectrophotometer Specord M-40 (Carl Zeiss, Germany).
The content of chlorophyll a, chlorophyll b, and carotenoids in wheat leaves was calculated in accordance with formula [41,42]:
Cchl a = 13.36 × A664.2 – 5.19 × A648.6,
Cchl b = 27.43 × A648.6 – 8.12A × 664.2,
Cchl (a + b) = 5.24 × A664.2 + 22.24 × A648.6,
Ccar = (1000 × A470 – 2.13 × Cchl a – 97.64 × Cchlb)/209,
Where,
Cchl – concentration of chlorophylls (µg/ml), Cchl a – concentration of chlorophyll a (µg/ml), Cchl b – concentration of chlorophyll b (µg/ml), Ccar – concentration of carotenoids (µg/ml), А – absorbance value at a proper wavelength in nm.
The chlorophyll and carotenoids content per 1 g of Fresh Weight (FW) extracted from wheat leaves was calculated by the following formula (separately for chlorophyll a, chlorophyll b, and carotenoids):
A1 = (C × V)/(1000 × a1),
Where, A1 – content of chlorophyll a, chlorophyll b, or carotenoids (mg/g FW),
C - Concentration of pigments (µg/ml),
V - Volume of extract (ml),
a1 - Sample of wheat leaves (g).
The content of chlorophyll a, chlorophyll b, and carotenoids (%) determined in experimental wheat leaves was calculated according to similar parameters determined in control wheat leaves.
Statistical data analysis
Each experiment was performed three times. Statistical processing of the experimental data was carried out using Student’s t-test with a significance level of p ≤ 0.05; mean values ± standard deviation (± SD) [43].
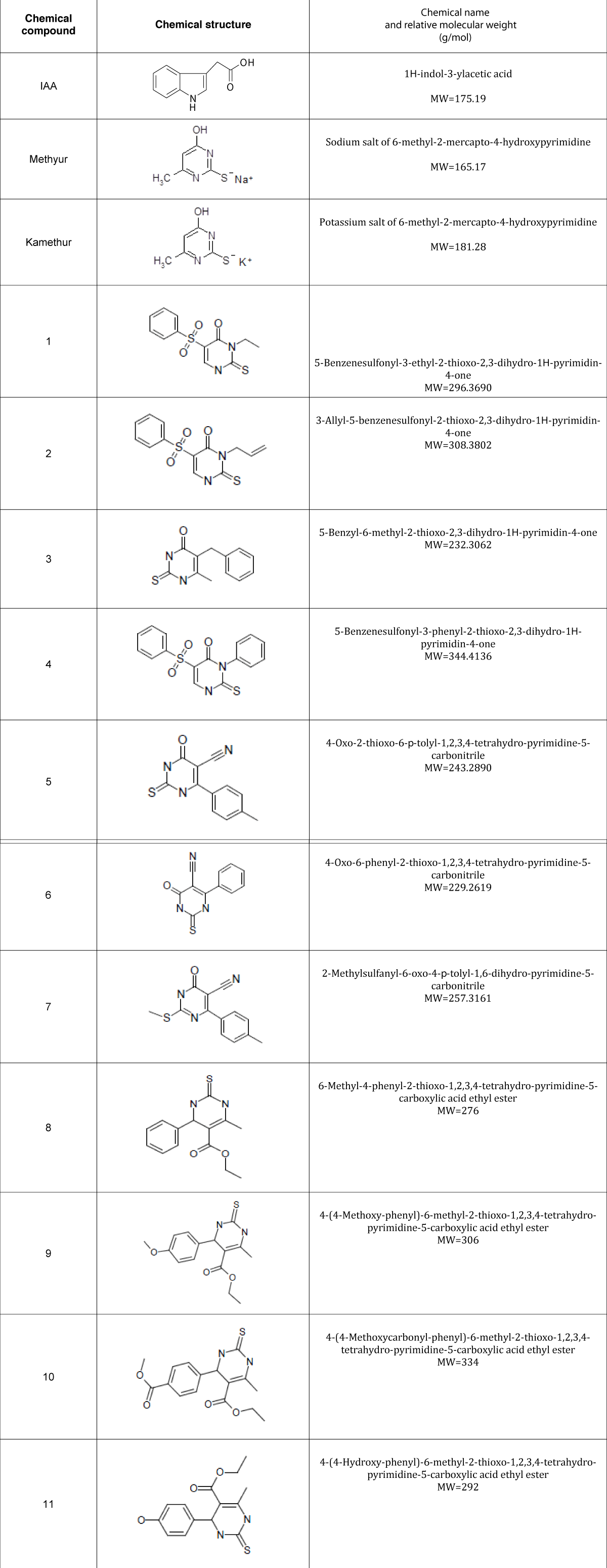
The regulatory effect of new synthetic thienopyrimidine derivatives compared to the regulatory effect of auxin IAA and synthetic plant growth regulators Methyur and Kamethur on the vegetative growth of wheat (Triticum aestivum L.) variety Svitlana was studied. The morphometric parameters of wheat plants grown from seeds treated with synthetic thienopyrimidine derivatives (compounds № 1 – 11, Table 1), auxin IAA, and synthetic plant growth regulators Methyur and Kamethur at a concentration of 10-6M, measured after 2 weeks were compared with similar parameters of control wheat plants grown from seeds treated with distilled water. The obtained results show that the growth-regulatory effect of new synthetic thienopyrimidine derivatives was similar to the growth-regulatory effect of auxin IAA or synthetic plant growth regulators Methyur and Kamethur (Figure 1).
Figure 1: The regulatory effect of auxin IAA, synthetic plant growth regulators Methyur, Kamethur, and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) at a concentration of 10-6M on the growth of shoots and roots of 2-week-old wheat (Triticum aestivum L.) variety Svitlana compared to control plants.
Increased growth and development of shoots and roots of wheat plants was observed for 2 weeks compared to control plants (Figure 1).
The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with auxin IAA at a concentration of 10-6M, statistically significantly increased by 32,67%, respectively, compared to control plants (Figure 2).
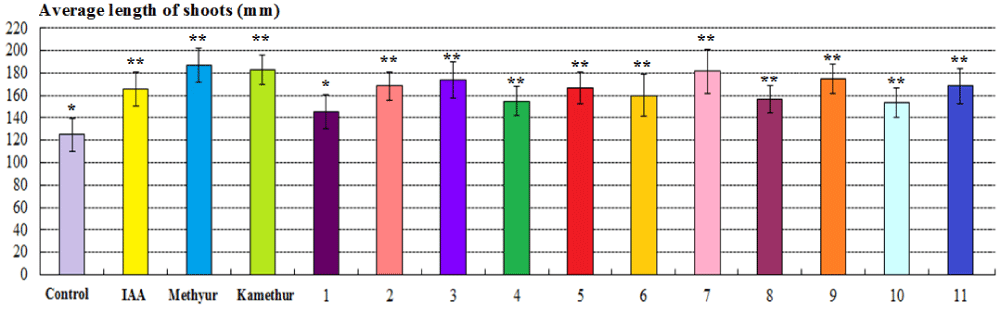
Figure 2: The regulatory effect of auxin IAA, synthetic plant growth regulators Methyur, Kamethur, and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) at a concentration of 10-6M on the average length of shoots (mm) of 2-week-old wheat (Triticum aestivum L.) variety Svitlana compared to control plants. Note. **Significant differences from control values*, p ≤ 0.05, n = 3, the values are mean ± SD.
The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with plant growth regulator Methyur at a concentration of 10-6M, statistically significantly increased by 49,33%, respectively, compared to control plants (Figure 2). The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with plant growth regulator Kamethur at a concentration of 10-6M, statistically significantly increased by 46,67%, respectively, compared to control plants (Figure 2). The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with the most active synthetic thienopyrimidine derivatives № 2, 3, 5, 7, 9 and 11 at a concentration of 10-6M, statistically significantly increased by 33,33% – 45,33%, respectively, compared to control plants (Figure 2). The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with the less active synthetic thienopyrimidine derivatives № 4, 6, 8 and 10 at a concentration of 10-6M, statistically significantly increased by 24% - 28%, respectively, compared to control plants (Figure 2). The parameters of the average length of shoots of 2-week-old wheat plants grown from seeds treated with the less active synthetic thienopyrimidine derivative № 1 at a concentration of 10-6M, increased by 16,67%, but did not differ statistically significantly from control plants (Figure 2).
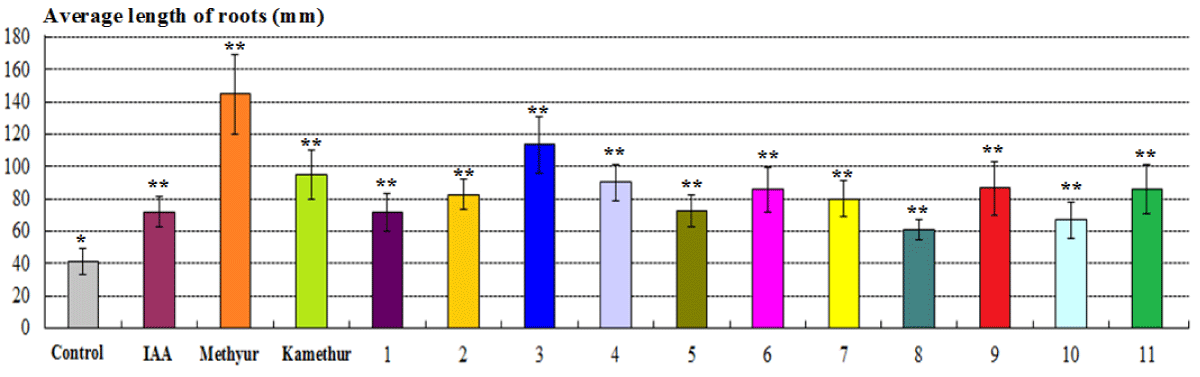
The parameters of the average length of roots of 2-week-old wheat plants grown from seeds treated with auxin IAA at a concentration of 10-6M, statistically significantly increased by 75,51%, respectively, compared to control plants (Figure 3). The parameters of the average length of roots of 2-week-old wheat plants grown from seeds treated with plant growth regulator Methyur at a concentration of 10-6M, statistically significantly increased by 255,1%, respectively, compared to control plants (Figure 3). The parameters of the average length of roots of 2-week-old wheat plants grown from seeds treated with plant growth regulator Kamethur at a concentration of 10-6M, statistically significantly increased by 132,65%, respectively, compared to control plants (Figure 3). The parameters of the average length of roots of 2-week-old wheat plants grown from seeds treated with the most active synthetic thienopyrimidine derivatives № 2 – 4, 6, 7, 9 and 11 at a concentration of 10-6M, statistically significantly increased by 95,92% – 177,55%, respectively, compared to control plants (Figure 3). The parameters of the average length of roots of 2-week-old wheat plants grown from seeds treated with the less active synthetic thienopyrimidine derivatives № 1, 5, 8 and 10 at a concentration of 10-6M, statistically significantly increased by 48,98% – 75,51 %, respectively, compared to control plants (Figure 3).
Figure 3: The regulatory effect of auxin IAA, synthetic plant growth regulators Methyur, Kamethur, and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) at a concentration of 10-6M on the average length of roots (mm) of 2-week-old wheat (Triticum aestivum L.) variety Svitlana compared to control plants. Note. **Significant differences from control values*, p ≤ 0.05, n = 3, the values are mean ± SD.
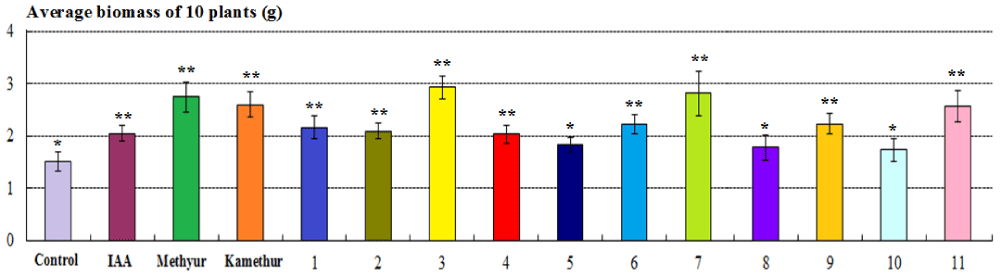
The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with auxin IAA at a concentration of 10-6M, statistically significantly increased by 35,16%, respectively, compared to control plants (Figure 4). The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with plant growth regulator Methyur at a concentration of 10-6M, statistically significantly increased by 81,31%, respectively, compared to control plants (Figure 4). The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with plant growth regulator Kamethur at a concentration of 10-6M, statistically significantly increased by 71,43%, respectively, compared to control plants (Figure 4). The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with the most active synthetic thienopyrimidine derivatives № 3, 6, 7, 9 and 11 at a concentration of 10-6M, statistically significantly increased by 46,15% – 93,41%, respectively, compared to control plants (Figure 4). The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with the less active synthetic thienopyrimidine derivatives № 1, 2 and 4, at a concentration of 10-6M, statistically significantly increased by 34,07% – 42,86%, respectively, compared to control plants (Figure 4). The parameters of the average biomass of 10 plants (g) of 2-week-old wheat plants grown from seeds treated with the less active synthetic thienopyrimidine derivatives № 5, 8 and 10 at a concentration of 10-6M, increased by 14,29% – 20,88%, but did not differ statistically significantly from control plants (Figure 4).
Figure 4: The regulatory effect of auxin IAA, synthetic plant growth regulators Methyur, Kamethur, and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) at a concentration of 10-6M on the average biomass of 10 plants (g) of 2-week-old wheat (Triticum aestivum L.) variety Svitlana compared to control plants. Note. **Significant differences from control values*, p ≤ 0.05, n = 3, the values are mean ± SD.
Summarizing the obtained morphometric parameters of wheat plants (average length of shoots and roots (mm), average biomass of 10 plants (g)), it should be noted that new synthetic thienopyrimidine derivatives (compounds № 2, 3, 6, 7, 9 and 11) showed the highest growth-regulatory effect, which was similar or higher than that of the auxin IAA or the synthetic plant growth regulators Methyur and Kamethur.
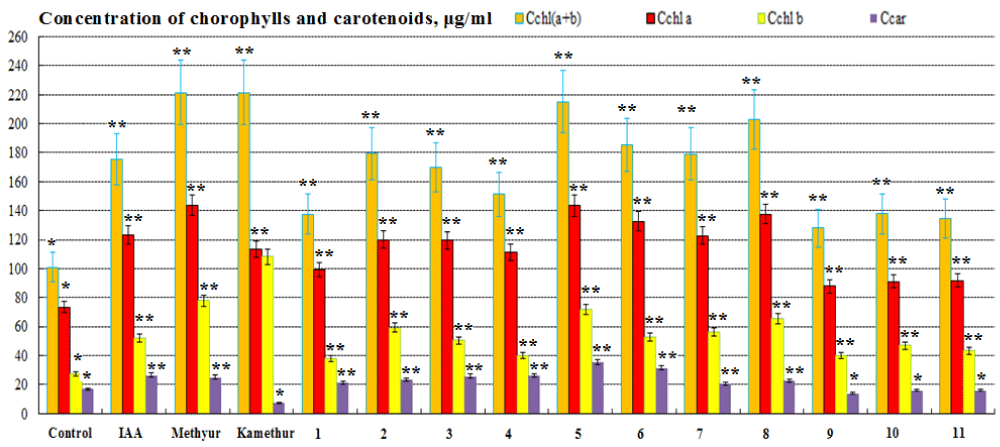
The regulatory effect of new synthetic thienopyrimidine derivatives (compounds № 1 – 11, Table 1), auxin IAA, and synthetic plant growth regulators Methyur and Kamethur at a concentration of 10-6M on the content of photosynthetic pigments (chlorophyll a, chlorophyll b, chlorophylls a+b, and carotenoids) in the leaves of 2-week-old wheat (Triticum aestivum L.) variety Svitlana was also studied. The content of photosynthetic pigments in the leaves of 2-week-old wheat plants grown from seeds treated with auxin IAA at a concentration of 10-6M, statistically significantly increased: chlorophyll a – by 67,72%, chlorophyll b - by 89,7%, chlorophylls a+b - by 73,7%, carotenoids - by 56,7%, respectively, compared to control plants (Figure 5). The content of photosynthetic pigments in the leaves of 2-week-old wheat plants grown from seeds treated with plant growth regulator Methyur at a concentration of 10-6M, statistically significantly increased: chlorophyll a – by 95,56 %, chlorophyll b - by 183,22%, chlorophylls a+b - by 119,42%, carotenoids - by 47,9%, respectively, compared to control plants (Figure 5). The content of photosynthetic pigments in the leaves of 2-week-old wheat plants grown from seeds treated with plant growth regulator Kamethur at a concentration of 10-6M, statistically significantly increased: chlorophyll a - by 54,25%, chlorophyll b - by 293,63%, chlorophylls a+b - by 119,42%, respectively, compared to control plants (Figure 5). The content of photosynthetic pigments in the leaves of 2-week-old wheat plants grown from seeds treated with the most active synthetic thienopyrimidine derivatives № 2 – 8 at a concentration of 10-6M, statistically significantly increased: chlorophyll a - by 51,54% – 95,21%, chlorophyll b - by 45,29% – 160,8%, chlorophylls a+b - by 49,84% – 113,07%, carotenoids - by 23,3% – 108,38%, respectively, compared to control plants (Figure 5). The content of photosynthetic pigments in the leaves of 2-week-old wheat plants grown
from seeds treated with the less active synthetic thienopyrimidine derivatives № 1, 9, 10 and 11 at a concentration of 10-6M, statistically significantly increased: chlorophyll a – by 19,43% – 35,49%, chlorophyll b – by 37,79% – 70,61%, chlorophylls a+b - by 26,72% – 36,67%, respectively, compared to control plants (Figure 5). The content of carotenoids in the leaves of 2-week-old wheat plants grown from seeds treated with synthetic thienopyrimidine derivative № 1 at a concentration of 10-6M, statistically significantly increased by 24,88%, respectively, compared to control plants (Figure 5). At the same time, the content of carotenoids in the leaves of 2-week-old wheat plants grown from seeds treated with plant growth regulator Kamethur and synthetic thienopyrimidine derivatives № 9, 10 and 11 at a concentration of 10-6M, did not differ statistically significantly from control plants (Figure 5).
Figure 5: The regulatory effect of auxin IAA, synthetic plant growth regulators Methyur, Kamethur, and new synthetic thienopyrimidine derivatives (compounds № 1 – 11) at a concentration of 10-6M on the content of chlorophylls a, b, and carotenoids (µg/ml) in the leaves of 2-week-old wheat (Triticum aestivum L.) variety Svitlana compared to control plants. Note. **Significant differences from control values*, p ≤ 0.05, n = 3, the values are mean ± SD.
Thus, the obtained results confirmed the positive regulatory effect of synthetic thienopyrimidine derivatives (compounds № 2 – 8) at a concentration of 10-6M on increasing the content of chlorophylls a, b, and carotenoids (µg/ml) in the leaves of 2-week-old wheat (Triticum aestivum L.) variety Svitlana, which play a key role in photosynthesis and ensuring plant productivity [41,42]. The regulatory effect of synthetic thienopyrimidine derivatives (compounds № 2 – 8) was similar to or higher than that of the auxin IAA or the synthetic plant growth regulators Methyur and Kamethur.
Analyzing the relationship between the chemical structure and biological activity of new most active synthetic thienopyrimidine derivatives № 2, 3, 5 – 9 and 11, it can be assumed that the high growth-regulatory effect of these compounds is associated with the presence of substituents in their chemical structure (Table 1): compound № 2 contains an allyl substituent in position 3, a phenylsulfonyl group in position 5 of the 2-thioxo-2,3-dihydro-1H-pyrimidin-4-one ring; compound № 3 contains a benzyl substituent in position 5, a methyl group in position 6 of the 2-thioxo-2,3-dihydro-1H-pyrimidin-4-one ring; compound № 5 contains a p-tolyl group in position 6, a cyano group in position 5 of the 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine ring; compound № 6 contains a phenyl group in position 6, a cyano group in position 5 of the 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine ring; compound № 7 contains a methylsulfanyl group in position 2, a p-tolyl group in position 4, and a cyano group in position 5 of the 6-oxo-1,6-dihydropyrimidine ring; compound № 8 contains a methyl group in position 6, a phenyl group in position 4, and an ethoxycarbonyl group in position 5 of the 2-thioxo-1,2,3,4-tetrahydropyrimidine ring; compound № 9 contains a methyl group in position 6, a 4-methoxyphenyl group in position 4, and an ethoxycarbonyl group in position 5 of the 2-thioxo-1,2,3,4-tetrahydropyrimidine ring; compound № 11 contains a methyl group in position 6, a 4-hydroxyphenyl group in position 4, and an ethoxycarbonyl group in position 5 of the 2-thioxo-1,2,3,4-tetrahydropyrimidine ring.
At the same time, the decrease in the growth-regulatory effect of synthetic thienopyrimidine derivatives № 1, 4, and 10 can be explained by the presence of substituents in the chemical structures of these compounds (Table 1): compound № 1 contains a benzenesulfonyl group in position 5, an ethyl group in position 3 of the 2-thioxo-2,3-dihydro-1H-pyrimidin-4-one ring; compound № 4 contains a phenyl group in position 3, a benzenesulfonyl group in position 5 of the 2-thioxo-2,3-dihydro-1H-pyrimidin-4-one ring; compound № 10 contains a methyl group in position 6, a 4-methoxycarbonylphenyl group in position 4, and an ethoxycarbonyl group in position 5 of the 2-thioxo-1,2,3,4-tetrahydropyrimidine ring.
Summarizing the obtained morphometric and biochemical parameters of wheat plants, it should be noted that the regulatory effect of the new most active synthetic thienopyrimidine derivatives № 2, 3, 5 – 9, and 11 were similar or higher than that of the auxin IAA or the synthetic plant growth regulators Methyur and Kamethur.
Based on data from studying the physiological and molecular mechanisms of signal transduction of natural auxins and cytokinins and their synthetic physiological analogues, it can be assumed that the growth-regulating effect of thienopyrimidine derivatives is explained by both their influence on the pathways of perception and transmission of natural auxin and cytokinin signals and their influence on the pathways of biosynthesis, metabolism, and transport of natural auxins and cytokinins in plant cells, which regulate the growth and development of plant shoots and roots and also slow down the degradation of chlorophylls and carotenoids in plant cells [1-13,44-60].
The obtained results confirmed the possibility of practical use of the most active thienopyrimidine derivatives (compounds № 2, 3, 5 – 9, and 11) at a concentration of 10-6M as new synthetic physiological analogues of auxins and cytokinins for the regulation of growth and development of wheat (Triticum aestivum L.) variety Svitlana in the vegetative phase and increasing the content of photosynthetic pigments in plant leaves, which ensure plant productivity.
- Su YH, Liu YB, Zhang XS. Auxin-cytokinin interaction regulates meristem development. Mol Plant. 2011 Jul;4(4):616-25. doi: 10.1093/mp/ssr007. Epub 2011 Feb 28. PMID: 21357646; PMCID: PMC3146736.
- Schaller GE, Bishopp A, Kieber JJ. The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell. 2015 Jan;27(1):44-63. doi: 10.1105/tpc.114.133595. Epub 2015 Jan 20. PMID: 25604447; PMCID: PMC4330578.
- Raggi S, Doyle SM, Robert S. Auxin: At the Crossroads Between Chemistry and Biology. Chemical Biology of Plant Biostimulants (Eds D. Geelen and L. Xu). Book Series Wiley Series in Renewable Resources. 2020;122-152. https://doi.org/10.1002/9781119357254.ch5
- Sosnowski J, Truba M, Vasileva V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture. 2023; 13(3): 724. https://doi.org/10.3390/agriculture13030724.
- Novickienė L, Asakavičiūtė R. Analogues of auxin modifying growth and development of some monocot and dicot plants. Acta Physiol Plant. 2006; 28(6): 509 – 515. https://doi.org/10.1007/s11738-006-0046-6.
- Simon S, Kubeš M, Baster P, Robert S, Dobrev PI, Friml J, Petrášek J, Zažímalová E. Defining the selectivity of processes along the auxin response chain: a study using auxin analogues. New Phytol. 2013 Dec;200(4):1034-48. doi: 10.1111/nph.12437. Epub 2013 Aug 5. PMID: 23914741.
- Rigal A, Ma Q, Robert S. Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci. 2014 Jul 30;5:373. doi: 10.3389/fpls.2014.00373. PMID: 25126092; PMCID: PMC4115670.
- Paque S, Weijers D. Q&A: Auxin: the plant molecule that influences almost anything. BMC Biol. 2016 Aug 10;14(1):67. doi: 10.1186/s12915-016-0291-0. PMID: 27510039; PMCID: PMC4980777.
- Ma Q, Grones P, Robert S. Auxin signaling: a big question to be addressed by small molecules. J Exp Bot. 2018 Jan 4;69(2):313-328. doi: 10.1093/jxb/erx375. PMID: 29237069; PMCID: PMC5853230.
- Vylíčilová H, Bryksová M, Matušková V, Doležal K, Plíhalová L, Strnad M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules. 2020 May 29;10(6):832. doi: 10.3390/biom10060832. PMID: 32485963; PMCID: PMC7356397.
- Jameson PE. Zeatin: The 60th anniversary of its identification. Plant Physiol. 2023 May 2;192(1):34-55. doi: 10.1093/plphys/kiad094. PMID: 36789623; PMCID: PMC10152681.
- Podlešáková K, Zalabák D, Cudejková M, Plíhal O, Szüčová L, Doležal K, Spíchal L, Strnad M, Galuszka P. Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS One. 2012;7(6):e39293. doi: 10.1371/journal.pone.0039293. Epub 2012 Jun 18. PMID: 22723989; PMCID: PMC3377648.
- Naseem A, Mohammad F. Thidiazuron: From Urea Derivative to Plant Growth Regulator. Singapore: Springer. 2018; 491. https://lib.ugent.be/catalog/ebk01:4100000002892653
- Tsygankova VA, Andrusevich YaV, Vasylenko NM, Pilyo SG, Klyuchko SV, Brovarets VS. Screening of Auxin-like Substances among Synthetic Compounds, Derivatives of Pyridine and Pyrimidine. J Plant Sci Phytopathol. 2023; 7: 151-156. DOI: 10.29328/journal.jpsp.1001121.
- Tsygankova VA, Andrusevich YaV, Shtompel OI, Kopich VM, Solomyanny RM, Brovarets VS. Study of regulating activity of synthetic low molecular weight heterocyclic compounds, derivatives of pyrimidine on growth of tomato (Solanum lycopersicum L.) seedlings, International Journal of ChemTech Research. 2019; 12(5): 26-38. http://dx.doi.org/10.20902/IJCTR.2019.120504
- Tsygankova VA, Voloshchuk IV, Andrusevich YaV, Kopich VM, Pilyo SG, Klyuchko SV, Kachaeva MV, Brovarets VS. Pyrimidine derivatives as analogues of plant hormones for intensification of wheat growth during the vegetation period. Journal of Advances in Biology. 2022; 15: 1-10. https://doi.org/10.24297/jab.v15i.9237.
- TsygankovaV A, Andrusevich YaV, Shtompel OI, Solomyanny RM, Hurenko AO, Frasinyuk MS, Mrug GP, Shablykin OV, Pilyo SG, Kornienko AM, Brovarets VS. New Auxin and Cytokinin Related Compounds Based on Synthetic Low Molecular Weight Heterocycles, Chapter 16, In: Aftab T. (Ed.) Auxins, Cytokinins and Gibberellins Signaling in Plants, Signaling and Communication in Plants, Springer Nature Switzerland AG. 2022; 353-377. DOI: https://doi.org/10.1007/978-3-031-05427-3_16.
- Tsygankova VA, Voloshchuk IV, Kopich VM, Pilyo SG, Klyuchko SV, Brovarets VS. Studying the effect of plant growth regulators Ivin, Methyur and Kamethur on growth and productivity of sunflower. Journal of Advances in Agriculture. 2023; 14:17-24. https://doi.org/10.24297/jaa.v14i.9453.
- Tsygankova VA, Voloshchuk IV, Andrusevich YaV, Kopich VM, Oliynyk OO, Stefanovska TR, Pidlisnyuk V, Pilyo SG, Klyuchko SV, Brovarets VS. Use of synthetic plant growth regulators in agriculture and biotechnology. Polish Journal of Science. 2023; 1(68):12-17. DOI: 10.5281/zenodo.10131991.
- Tsygankova VA, Voloshchuk IV, Pilyo SH, Klyuchko SV, Brovarets VS. Enhancing Sorghum Productivity with Methyur, Kamethur, and Ivin Plant Growth Regulators. Biology and Life Sciences Forum. 2023; 27(1):36. https://doi.org/10.3390/IECAG2023-15222.
- Tsygankova VA, Andrusevich YaV, Kopich VM, Voloshchuk IV, Bondarenko OM, Pilyo SG, Klyuchko SV, Brovarets VS. Effect of pyrimidine and pyridine derivatives on the growth and photosynthesis of pea microgreens. Int J Med Biotechnol Genetics. 2023; S1:02:003:15-22. https://scidoc.org/IJMBGS1V2.php.
- Tsygankova VA, Kopich VM, Vasylenko NM, Andrusevich YaV, Pilyo SG, Brovarets VS. Phytohormone-like effect of pyrimidine derivatives on the vegetative growth of haricot bean (Phaseolus vulgaris L.). Polish Journal of Science. 2024; 1(71):6-13. DOI: 10.5281/zenodo.10675232.
- El-Sherbeny MA, El-Ashmawy MB, El-Subbagh HI, El-Emam AA, Badria FA. Synthesis, antimicrobial and antiviral evaluation of certain thienopyrimidine derivatives. European Journal of Medicinal Chemistry. 1995; 30(5):445-449. https://doi.org/10.1016/0223-5234(96)88255-9.
- Bhuiyan MD, Rahman KM, Hossain MD, Rahim A, Hossain MI, Abu Naser M. Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives. Acta Pharm. 2006 Dec;56(4):441-50. PMID: 19839136.
- Abdel-Megid M, Elmahdy KM, Elkazak AM, Seada MH, Mohamed OF. Chemistry of Thienopyrimidines and Their Biological Applications. J. Pharm. Appl. Chem. 2016; 2(3):103-127. http://dx.doi.org/10.18576/jpac/020301.
- Tolba MS, Ahmed M, Kamal El‐Dean AM, Hassanien R, Farouk M. Synthesis of New Fused Thienopyrimidines Derivatives as Anti‐inflammatory Agents. Journal of Heterocyclic Chemistry. 2017; 55(2):408-418. https://doi.org/10.1002/jhet.3056.
- Tolba MS, El-Dean KAM, Ahmed M, Hassanien R. Synthesis, reactions, and biological study of some new thienopyrimidine derivatives as antimicrobial and anti-inflammatory agents. Journal of the Chinese Chemical Society. 2019; 66(5):548-557. https://doi.org/10.1002/jccs.201800292.
- Lagardère P, Fersing C, Masurier N, Lisowski V. Thienopyrimidine: A Promising Scaffold to Access Anti-Infective Agents. Pharmaceuticals (Basel). 2021 Dec 27;15(1):35. doi: 10.3390/ph15010035. PMID: 35056092; PMCID: PMC8780093.
- Sayed MTM, Hassan RA, Halim PA, El-Ansary AK. Recent updates on thienopyrimidine derivatives as anticancer agents. Med Chem Res. 2023; 32(4):659-681. https://doi.org/10.1007/s00044-023-03040-y.
- Vlasova OD, Vlasov SV, Kabachnyy VI, Vlasov VS. The Synthesis, Transformations and Biological Activity of thieno[2,3-d]pyrimidine Derivatives With the Carboxylic Groups As the Substituents in the Pyrimidine Ring. J. Org. Pharm. Chem. 2020; 18:4-13. DOI: https://doi.org/10.24959/ophcj.20.209835.
- Ota C, Kumata S, Kawaguchi S. Novel herbicides, usage thereof, novel thienopyrimidine derivatives, intermediates of the same, and process for production thereof. Patent US20070010402A1. 2007.
- Wilding B, Faschauner S, Klempier N. A practical synthesis of 5-functionalizedthieno[2,3-d]pyrimidines. Tetrahedron Lett. 2015; 56(30):4486-4489. DOI: 10.1016/j.tetlet.2015.05.104.
- Abdel-Megid M, Elmahdy KM, Elkazak AM, Seada MH, Mohamed OF. Chemistry of Thienopyrimidines and Their Biological Applications. J. Pharm. Appl. Chem. 2016; 2(3):103-127. http://dx.doi.org/10.18576/jpac/020301.
- Wang DW, Li Q, Wen K, Ismail I, Liu DD, Niu CW, Wen X, Yang GF, Xi Z. Synthesis and Herbicidal Activity of Pyrido[2,3-d]pyrimidine-2,4-dione-Benzoxazinone Hybrids as Protoporphyrinogen Oxidase Inhibitors. J Agric Food Chem. 2017; 65(26):5278 - 5286. DOI: 10.1021/acs.jafc.7b01990.
- El-Dean AMK, Abd-Ella AA, Hassanien R, El-Sayed MEA, Zaki RM, Abdel-Raheem SAA. Chemical design and toxicity evaluation of new pyrimidothienotetrahydroisoquinolines as potential insecticidal agents. Toxicol Rep. 2018 Dec 18;6:100-104. doi: 10.1016/j.toxrep.2018.12.004. PMID: 30622903; PMCID: PMC6308254.
- Wang DW, Zhang H, Yu SY, Zhang RB, Liang L, Wang X, Yang HZ, Xi Z. Discovery of a Potent Thieno [2,3-d]pyrimidine-2,4-dione-Based Protoporphyrinogen IX Oxidase Inhibitor through an In Silico Structure-Guided Optimization Approach. J Agric Food Chem. 2021; 69(47):14115-14125. DOI: 10.1021/acs.jafc.1c05665.
- Li JH, Wang Y, Wu YP, Li RH, Liang S, Zhang J, Zhu YG, Xie BJ. Synthesis, herbicidal activity study and molecular docking of novel pyrimidine thiourea. Pestic Biochem Physiol. 2021 Feb;172:104766. doi: 10.1016/j.pestbp.2020.104766. Epub 2020 Dec 25. PMID: 33518053.
- Tolba MS, El-Dean KAM, Ahmed M, Sayed HRM, Zaki RM, Mohamed SK, Zawam SA, Abdel-Raheem SAA. Synthesis, reactions, and applications of pyrimidine derivatives. Current Chemistry Letters. 2022; 11:121-138. DOI: 10.1016/j.pestbp.2020.104766.
- Pivazyan VA, Ghazaryan EA, Karapetyan AV, Shainova RS, Harutyunyan SV, Vorskanyan AS, Yengoyan AP, Gomktsyan TA. Ultrasound-assisted green syntheses of novel pyrimidine derivatives and their comparison with conventional methods. Journal of Saudi Chemical Society. 2023; 27(3):101628. https://doi.org/10.1016/j.jscs.2023.101628.
- Voytsehovska OV, Kapustyan AV, Kosik OI. Plant Physiology: Praktykum, Parshikova T.V. (Ed.), Lutsk: Teren. 2010; 420.
- Lichtenthaler H. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology. 1987; 148:331-382.
- Lichtenthaler HK, Buschmann C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy Current Protocols in Food Analytical Chemistry (CPFA): John Wiley and Sons, New York. 2001; F4.3.1-F4.3.8.
- Bang H, Zhou XK, van Epps HL, Mazumdar M. (Eds.). Statistical Methods in Molecular Biology. Series: Methods in molecular biology, New York: Humana press. 2010; 13(620):636.
- Calderon-Villalobos LI, Tan X, Zheng N, Estelle M. Auxin perception--structural insights. Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a005546. doi: 10.1101/cshperspect.a005546. Epub 2010 May 26. PMID: 20504967; PMCID: PMC2890193.
- Ljung K. Auxin metabolism and homeostasis during plant development. Development. 2013 Mar;140(5):943-50. doi: 10.1242/dev.086363. PMID: 23404103.
- Lavy M, Estelle M. Mechanisms of auxin signaling. Development. 2016 Sep 15;143(18):3226-9. doi: 10.1242/dev.131870. PMID: 27624827; PMCID: PMC5047657.
- Leyser O. Auxin Signaling. Plant Physiol. 2018 Jan;176(1):465-479. doi: 10.1104/pp.17.00765. Epub 2017 Aug 17. PMID: 28818861; PMCID: PMC5761761.
- Pařízková B, Pernisová M, Novák O. What Has Been Seen Cannot Be Unseen-Detecting Auxin In Vivo. Int J Mol Sci. 2017 Dec 16;18(12):2736. doi: 10.3390/ijms18122736. PMID: 29258197; PMCID: PMC5751337.
- Fukui K, Hayashi KI. Manipulation and Sensing of Auxin Metabolism, Transport and Signaling. Plant Cell Physiol. 2018 Aug 1;59(8):1500-1510. doi: 10.1093/pcp/pcy076. PMID: 29668988.
- Blázquez MA, Nelson DC, Weijers D. Evolution of Plant Hormone Response Pathways. Annu Rev Plant Biol. 2020 Apr 29;71:327-353. doi: 10.1146/annurev-arplant-050718-100309. Epub 2020 Feb 4. PMID: 32017604.
- Casanova-Sáez R, Mateo-Bonmatí E, Ljung K. Auxin Metabolism in Plants. Cold Spring Harb Perspect Biol. 2021 Mar 1;13(3):a039867. doi: 10.1101/cshperspect.a039867. PMID: 33431579; PMCID: PMC7919392.
- Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annu Rev Plant Biol. 2012;63:353-80. doi: 10.1146/annurev-arplant-042811-105503. PMID: 22554243.
- Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018 Feb 27;145(4):dev149344. doi: 10.1242/dev.149344. PMID: 29487105.
- Sakakibara H. Cytokinins: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006; 57:431-449. DOI: 10.1146/annurev.arplant.57.032905.105231.
- Mok DW, Mok MC. CYTOKININ METABOLISM AND ACTION. Annu Rev Plant Physiol Plant Mol Biol. 2001 Jun;52:89-118. doi: 10.1146/annurev.arplant.52.1.89. PMID: 11337393.
- Hönig M, Plíhalová L, Husičková A, Nisler J, Doležal K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int J Mol Sci. 2018 Dec 14;19(12):4045. doi: 10.3390/ijms19124045. PMID: 30558142; PMCID: PMC6321018.
- Wu W, Du K, Kang X, Wei H. The diverse roles of cytokinins in regulating leaf development. Hortic Res. 2021 Jun 1;8(1):118. doi: 10.1038/s41438-021-00558-3. PMID: 34059666; PMCID: PMC8167137.
- Zhang YM, Guo P, Xia X, Guo H, Li Z. Multiple Layers of Regulation on Leaf Senescence: New Advances and Perspectives. Front Plant Sci. 2021 Dec 6;12:788996. doi: 10.3389/fpls.2021.788996. PMID: 34938309; PMCID: PMC8685244.
- Huang P, Li Z, Guo H. New Advances in the Regulation of Leaf Senescence by Classical and Peptide Hormones. Front Plant Sci. 2022 Jun 28;13:923136. doi: 10.3389/fpls.2022.923136. PMID: 35837465; PMCID: PMC9274171.
- Hu Y, Shani E. Cytokinin activity - transport and homeostasis at the whole plant, cell, and subcellular levels. New Phytol. 2023 Sep;239(5):1603-1608. doi: 10.1111/nph.19001. Epub 2023 May 27. PMID: 37243527.