More Information
Submitted: September 08, 2023 | Approved: September 12, 2023 | Published: September 13, 2023
How to cite this article: Shen J, He G, Tang X, Ren L, Fang B, et al. Analysis of Microbial Diversity and Community Structure in the Rhizosphere of Cigar Tobacco in Different Agroecological Zones. J Plant Sci Phytopathol. 2023; 7: 097-106.
DOI: 10.29328/journal.jpsp.1001113
Copyright License: © 2023 Shen J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Analysis of Microbial Diversity and Community Structure in the Rhizosphere of Cigar Tobacco in Different Agroecological Zones
Junru Shen1#, Guoyou He2#, Xubing Tang1*, Longhui Ren1, Bao Fang1, Anzhong Zhen1, Tao Yang2* and Chuisi Kong3*
1Yunnan Tobacco Baoshan Spice Tobacco Co., Ltd. 678000, China
2Biotechnology and Genetic Germplasm Institute, Yunnan Academy of Agricultural Sciences, Kunming 650205, China
3Agricultural Environment and Resources Institute, Yunnan Academy of Agricultural Sciences, Kunming 650205, China
*Address for Correspondence: Xubing Tang, Yunnan Tobacco Baoshan Spice Tobacco Co., Ltd. 678000, China, Email: [email protected]
Tao Yang, Biotechnology and Genetic Germplasm Institute, Yunnan Academy of Agricultural Sciences, Kunming 650205, China Email: [email protected]
Chuisi Kong, Agricultural Environment and Resources Institute, Yunnan Academy of Agricultural Sciences, Kunming 650205, China, Email: [email protected]
To reveal the influence of ecological zones on the structure of microbial communities in cigar rhizosphere soils, Yunnan's cigar tobacco production region was first divided into three ecological zones. Soil samples were collected at maturity and the community structure of fungi and bacteria in the rhizosphere soil was analyzed using 18S rRNA and 16S rRNA high-throughput sequencing techniques. The results showed that the dominant fungi were Ascomycota, Mortrellomycota, and Basidiomycota, and the dominant bacteria were Ascomycota and Proteobacteria. The dominant genera and relative abundances of fungi and bacteria differ at the genus level. Ecoregions may affect the community structure and distribution of fungal and bacterial diversity in the rhizospheric soil of cigars at maturity, which may provide a theoretical basis for the selection of high-quality cigar-producing regions in the future.
Tobacco is a thermophilic crop whose growth and development are influenced by genetic factors, ecological environment, and cultivation measures. Genetic factors are internal factors that affect the growth of tobacco plants, and climatic conditions are essential aspects of the ecological environment. Different climatic factors such as temperature, precipitation, and illumination in different production areas modify the soil conditions and spatial environment for tobacco growth, and then affect the growth and development of tobacco plants and their yield and quality [1], if the temperature is overly low, the tobacco plant grows slowly, the plant is short, and it is easy to "flower early"; when the temperature is overly steep, the respiration of tobacco plants is accelerated, the dry matter in the body is consumed, and heat injury is easily caused, which has adverse effects on the quality of tobacco leaves [2]. Cigar is a special tobacco product that features a strong, heavy aroma, and bitter and sweet taste and is less harmful to the human body than regular cigarettes due to less tar and nicotine. It is intensely loved by consumers at home and abroad and has great market potential [3]. Consumer groups and sales have continued to rise since cigars were introduced to China in the Qing Dynasty. Statistics show that production and sales of Chinese-style cigarettes have declined slightly, but production, sales, and sales of Chinese cigars have increased significantly, and cigars have become a new economic growth point for the Chinese tobacco industry [4]. However, the current shortage of raw materials for high-quality cigar tobacco, both at home and abroad, is gravely hampering the development of the high-quality cigar tobacco industry. Climatic conditions are critical in determining the quality of tobacco leaves. Temperature, light, and rainfall not only affect the growth of tobacco leaves in the field but also affect the appearance quality, physical characteristics, and chemical components of tobacco leaves [5]. However, the production of raw materials for different structural parts of cigars (wrapper, binder, and filler) has different and harsh ecological conditions [6]. For example, wrappers need to be planted under elevated temperatures, strong humidity, cloudy and less windy weather conditions, and different growth stages have different requirements for meteorological conditions [7,8]. For example, the temperature during the field growth period is required to be around 20 °C, while the temperature during the harvest period is required to exceed 22 °C and the relative air humidity is above 70% [9].
Soil microorganisms play an essential role in the ecosystem and are staple factors in soil quality, soil fertility, and productivity, and the diversity and community structure of soil microorganisms are directly related to soil-borne diseases and plant health [10]. The composition and structure of soil microbial communities in different ecological regions changed due to different biological and abiotic factors such as temperature, precipitation, and vegetation type [11]. In recent years, there have been preliminary studies on the variation of soil microorganisms under different ecological conditions using sequencing techniques. The results showed that there were significant differences in the structure construction, species composition, and driving factors of rice rhizosphere and non-rhizosphere bacterial communities in different ecological regions [12], different ecological types, climate types, and vegetation types affect the microbial community structure of soil in ecological areas, and it is found that the soil biological activity in Zhangjiajie tobacco-growing area of Hunan Province decreases with the increase of altitude [13,14].
Soil microorganisms are critical participants in ecosystem cycles, regulating the turnover of organic matter pools [15]. Using high-throughput sequencing technology to understand the unknown microbial groups and functions in soil, especially the diversity, community structure, and function of rhizosphere microorganisms, has played an essential role in the study of plant nutrient uptake mechanisms [16]. The ripening period of cigar tobacco is a critical period that affects the quality of the tobacco leaves, and rhizosphere soil microorganisms are one of the significant influences. In this study, high-throughput sequencing techniques were used to analyze the community diversity and composition of rhizosphere soil microorganisms under different ecological conditions in the Yunnan cigar smoking region. By studying the regulation mechanism of the rhizosphere soil microbial community by the Ecoregion, we can further clarify the understanding of the distribution of the soil microbial community structure, which will facilitate better prediction and analysis of the rhizosphere soil microbial community structure in the mature stage of cigar tobacco, and also provide insights into different Ecoregions. To provide a theoretical reference for micro-ecological regulation of cigar tobacco rhizosphere soil.
Overview of the test area
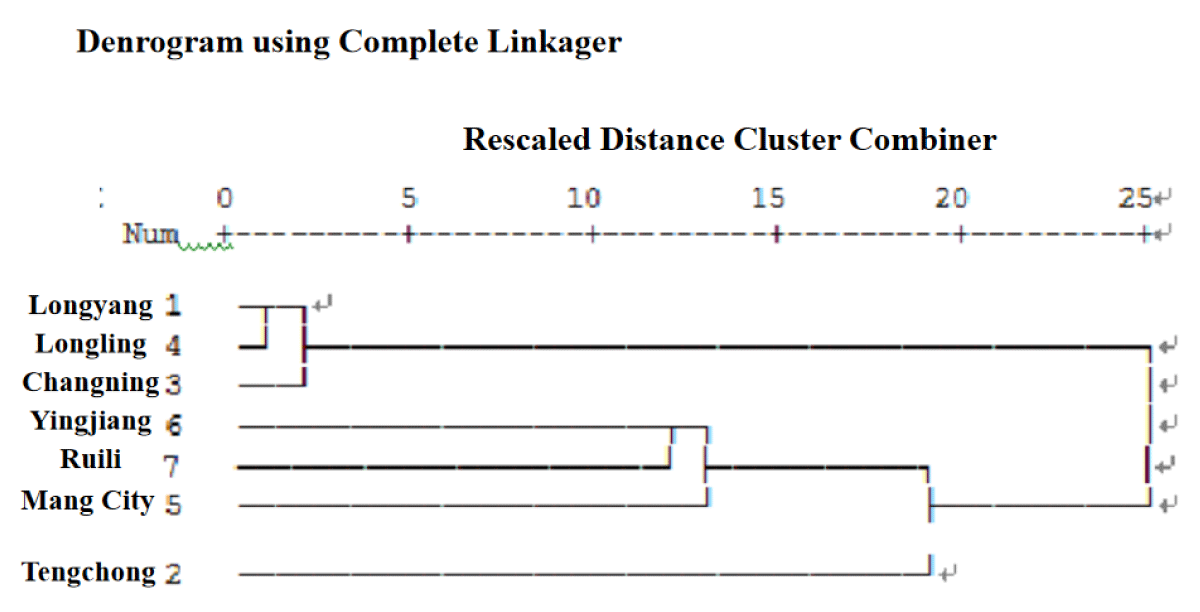
Yunxue No. 39 is provided by Yunnan Oriental Tobacco Baoshan Company. According to the meteorological data (ten-day temperature and ten-day rainfall) from 2017 to 2022, cluster analysis is carried out by using SPSS 20.0, and the Yunnan cigar tobacco area is divided into three ecological points(Figure 1): Ecological A (Longyang District, Longling, Changning), Ecological B (Yingjiang, Ruili, Mang City), Ecological C (Tengchong).
Figure 1: Cluster analysis of ecological regions according to meteorological data.
DNA extraction, PCR amplification and Illumina MiSeq sequencing
Fast DNA SPIN Kit for Soil (MP Biomedicals, LLC) was used to extract genomic DNA from a 0.5g soil sample, and then 1% agarose gel electrophoresis was used to detect the purity and concentration of DNA. A suitable amount of the sample was taken into a centrifuge tube. If the band is clear and complete, it can be used for subsequent testing.
Diluted genomic DNA was used as a template and PCR was performed using specific primers with Barcode and HiFi Hot Start Ready Mix high-fidelity enzymes from Yunnan Qingke Biological Co., Ltd. to ensure amplification efficiency and accuracy, depending on the selected sequencing region. PCR usage 50 μ L reaction system: 2 µL template DNA, 2 µL forward primer (10 µmol L-1), 2 µL reverse primer (10 µmol L-1), 4 µL dNTPs (2.5 µmol L-1), 5 µL 10 × Pyrobest buffer, 0.3 µL Pyrobest DNA polymerase (2.5 µL -1) and 34.7 µL ddH2O. PCR amplification reaction of fungal ITS: The primers used ITS F: (5'-CTTGGTCATTTAGAGAGGAAGTAA-3') and R: (5'-GCTGTGTTCATCGATGCCC-3'). PCR amplification reaction of bacterial 16S: The primers used 16S: F: (5'- ACTCCTACGGGGGCAGCA-3') and R: (5'-GACTACHVGGTWTC
TAAT-3'). PCR reaction procedure: pre-denaturation at 95 ℃ for 5 minutes; 95 ℃ denaturation for 30 seconds, 56 ℃ annealing for 30 seconds, 72 ℃ extension for 40 seconds, 30 cycles; extend at 72 ℃ for 10 minutes and detect PCR products by electrophoresis [17].
With reference to the preliminary quantitative results of electrophoresis, the PCR products were detected and quantified with Quanti Fluor™ -ST blue fluorescence quantitative system (Promega, Madison, WI, USA), and then mixed in corresponding proportion according to the sequencing quantity requirements of each sample. Sequencing was performed using the Illumina Miseq sequencing platform (Illumina, San Diego, CA, USA) of Yunnan Qingke Biological Co., Ltd. The pooled DNA products were used to construct Illumina paired-end libraries following Illumina's genomic DNA library preparation procedures. The amplicon library was then subjected to paired-end sequencing (2x250) on the Illumina MiSeq platform according to standard protocols.
Processing and analysis of sequencing data
Quality control was performed on the raw sequencing sequences, including low-quality filtering and length filtering, to obtain top-quality sequences. Clustering/denoising high-quality sequences, dividing them into OTUs/ASVs (later collectively referred to as Features), and obtaining their species classification based on the sequence composition of features; Based on the feature analysis results, a taxonomic analysis was performed on the samples at each classification level to obtain a community structure map for each sample at the taxonomic level of phylum, class, order, family, genus, and species. Species diversity within a single sample was studied through alpha diversity analysis, Ace, Chao, Shannon, and Simpson indexes of each sample were counted, and the sample dilution curve was drawn [18,19].
Fungal community composition of cigar tobacco in different ecoregions
The results of PCoA analysis of the rhizosphere soil fungal communities in the three Ecoregionis are shown in Figure 2a.
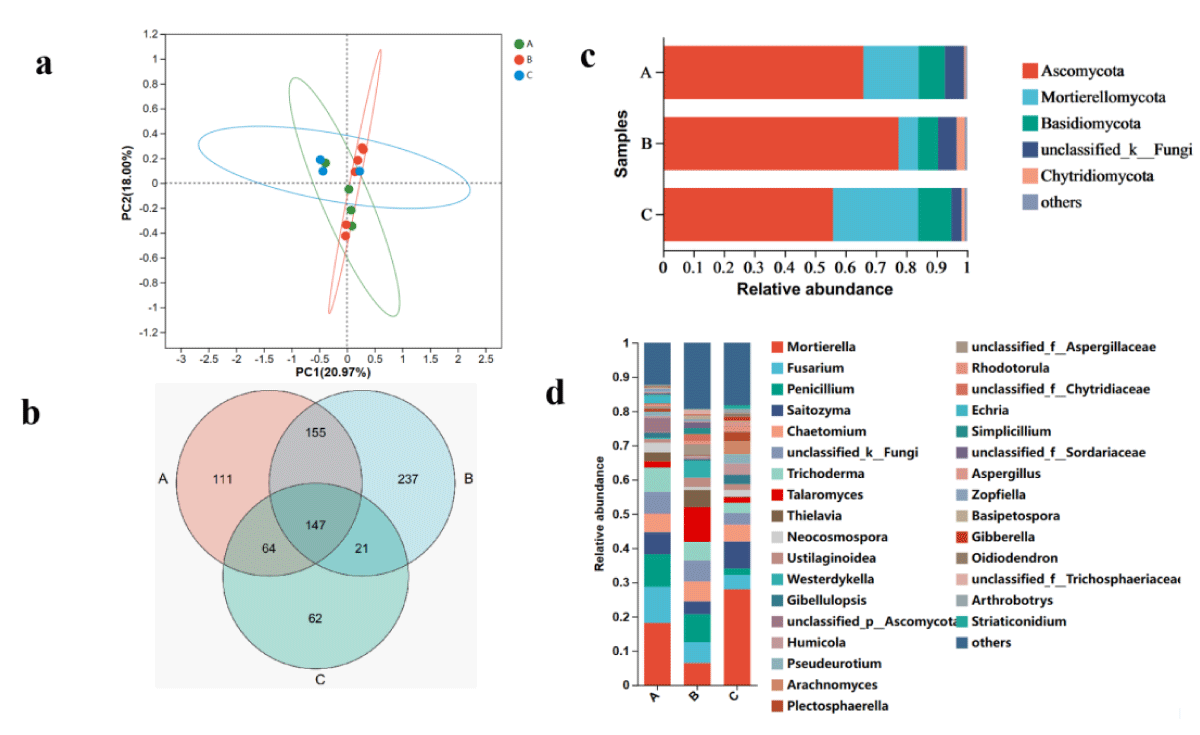
Figure 2: The microbiome compositions of fungal taxa in three soil samples. (a) UniFrac-weighted PCoA plots of fungal communities at phylum level in three samples. (b) Venn diagram of exclusive and shared fungal species in three soil samples. (c) Fungal community structure at the level of phylum. (d) The composition of the fungal community at the level of genus. A, ecology1; B, ecology2; C, ecology3; (phyla and genera with average relative abundance (RA) < 1% were merged and indicated as "Others").
The two principal coordinates account for 91.77% of the variation, with PC1 accounting for 20.97% of the variation and PC2 accounting for 18.00%. Furthermore, the PCoA analysis shows that the fungal taxa of the microbial communities in Ecozones A, B, and C share some similarities.
Metagenomic sequencing of three cigar soil samples revealed 896 fungal species belonging to 521 genera, 230 families, 103 orders, 44 classes, and 14 phyla. Ecozone A detected 478 species, Ecozone B detected 560 species, Ecozone C detected 294 species, and 147 species were common to all three populations (Figure 2b). Among them, the dominant fungal phyla in different ecological regions of cigar was Ascomycota, Mortierellomycota, Basidiomycota and unclassified_k_Fungi (RA > 1%) (Figure 2c). The total number of this phyla accounted for more than 91% of the fungal group, of which Ascomycota and Mortierellomycota accounted for the largest proportion (85.3%). In addition, the highest proportion of Ascomycota is found in Ecozone B, followed by Ecozone A and finally Ecozone C, but the opposite is true for Mortierellomycota.
At the genus level, Mortierella, Saitozyma, Penicillium, Saitozyma, and Saitozyma are the five most abundant fungal genera (RA > 2%). Mortierella and Saitozyma have the highest proportions in Ecoregion C; Saitozyma and Penicillium have the highest proportions in Ecoregion A; the proportion of Saitozyma is highest in Ecosystem B. It should be noted that different ecosystems have distinct effects on the structure of the fungal microbial community in the rhizosphere of cigar tobacco, which may provide a new theoretical basis for selecting suitable areas for cigar cultivation.
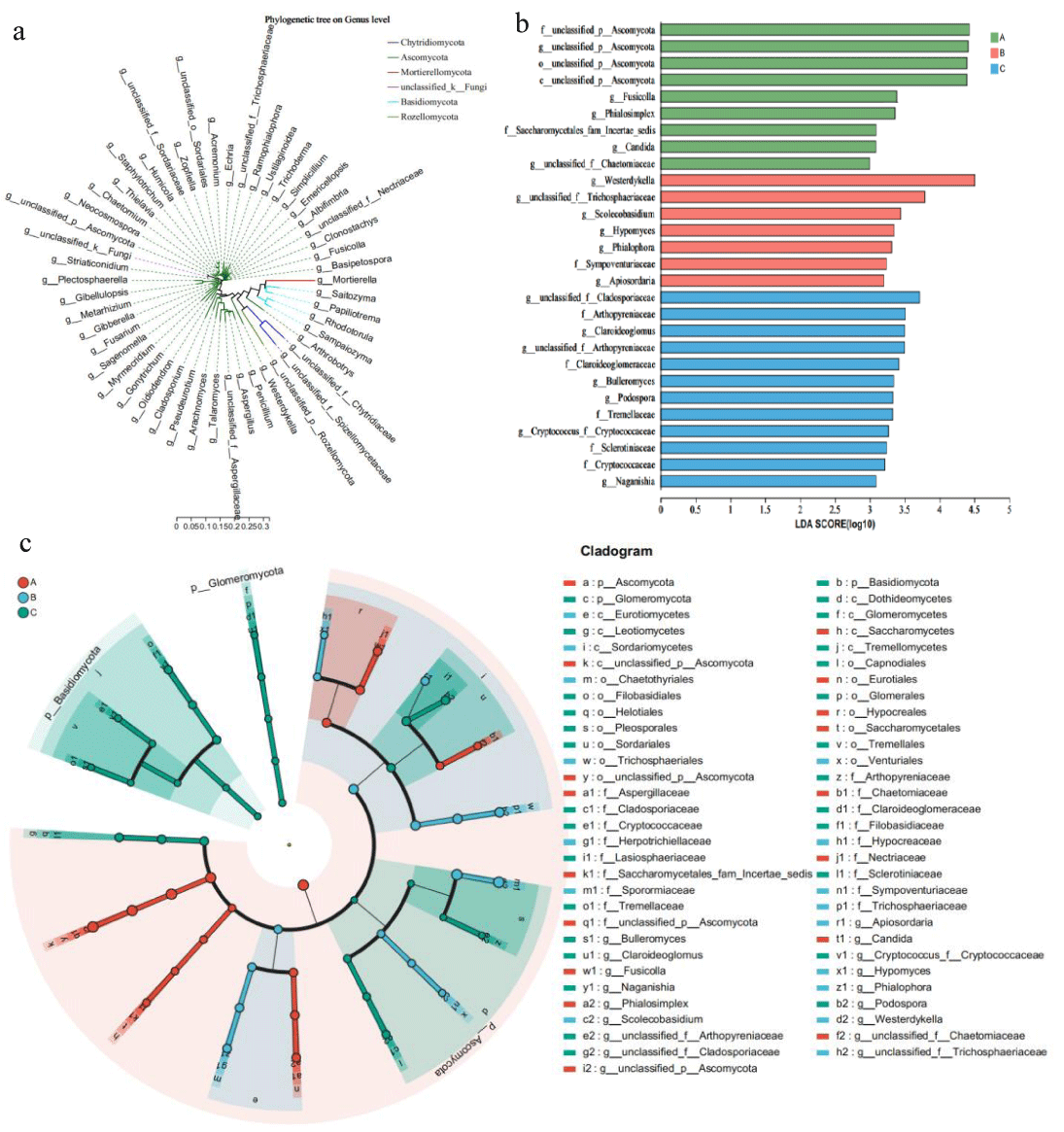
The phylogenetic relationships of the microbes detected by high-throughput sequencing indicated that the sum total of the cigar rhizosphere-associated microbial communities originated from various phyla (Figure 3). In order to further investigate which microorganisms are significantly enriched in the rhizosphere of cigar tobacco, LEfSe analysis was used. The results showed that Ascomycota was significantly enriched in Ecoregion A, while Arthopyreniaceae and Cladosporiaceae were significantly enriched in Ecoregion C. Westerdykella was significantly enriched in Ecoregion B. In conclusion, the observations in this paper suggest that different Ecoregions affect the enrichment of fungi in the cigar rhizosphere.
Figure 3: Comparison of microorganisms and fungi in the rhizosphere of cigar tobacco in different Ecoregions (a) Cladogram of 50 microbes (mean relative abundance) based on taxonomy. (b) LDA scores of biomarker fungal for each combination of cigar sites. LDA scores are shown as horizontal bars for the biomarker fungal with an LDA score > 4.8 as listed on the left, Kruskal–Wallis rank sum test, p < 0.05. LDA, linear discriminant analysis. (c) LEfSe analysis revealed distinct taxa of cigar tobacco rhizosphere fungi in different Ecoregions. Areas of different colors represent different components (red for Ecoregion 1, green for Ecoregion 2, green for Ecoregion 3), the diameter of each circle is proportional to the relative abundance of the taxa, and the inner circle to the outer ring corresponds to the phylum to the genus layer.
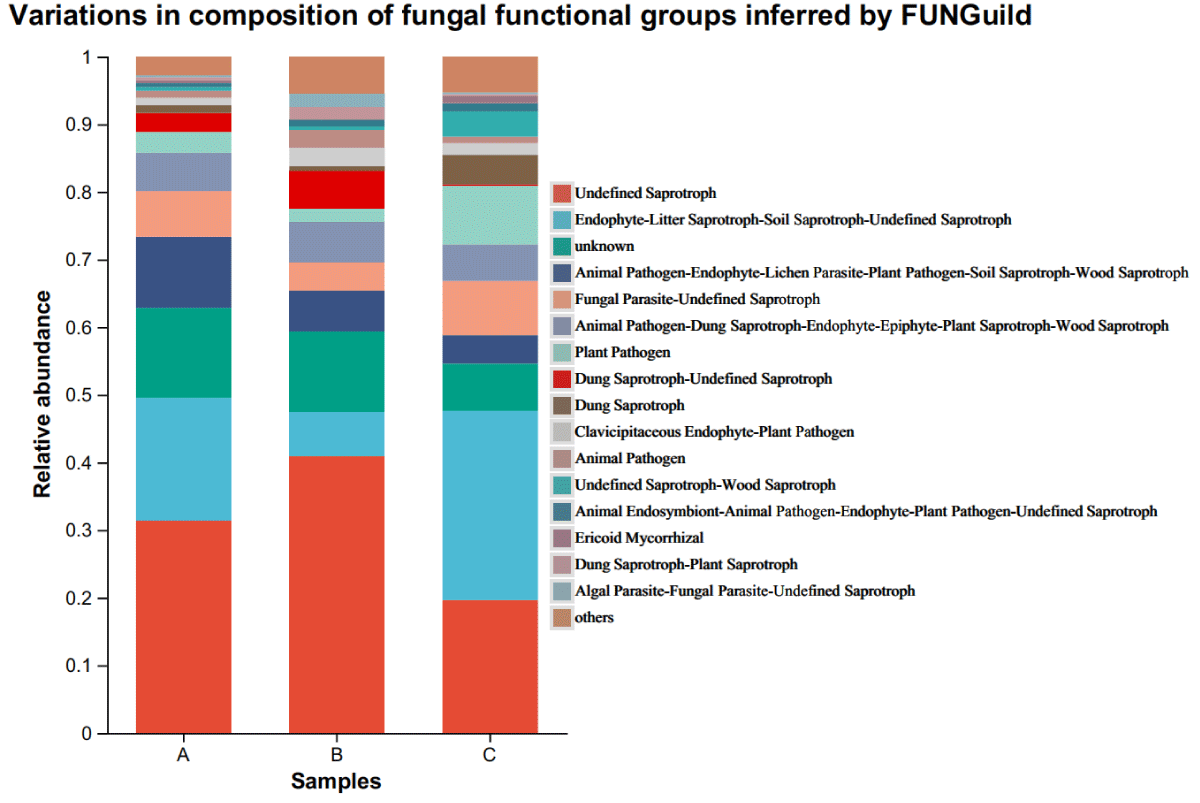
FUNGuild (Fungi Functional Guild) is a tool for taxonomic analysis of fungal communities through a microecological guild, a concept in microecology that refers to a group of species (whether closely related or not) that utilizes similar environmental resources in similar ways. Analysis of the function of rhizospheric microbial communities in different Ecoregions showed that groups with more than 1% dominant OTUs were divided into 16 functional groups, and groups with less than 1% dominant OTUs were merged into others. Different functional groups can be distinguished in three distinct ecological regions (Figure 4). The dominant functional groups of Ecozone A were Undefined Saprotroph (31.2%), Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph (17.5%), unknown (11.3%), animal pathogen-endophyte- lichen parasite-plant pathogen-wood Saprotroph (8.2%), Fungal parasite-Undefined Saprotroph (4.8%), animal Pathogen-Dung Saprotroph-Endophyte-Epiphyte-Plant Saprotroph-Wood Saprotroph (3.8%). Ecozone B dominant functional group was Undefined Saprotroph (38.3%), unknown (5.3%), Animal Pathogen-Dung Saprotroph-Endophyte-Epiphyte-Plant Saprotroph-Wood Saprotroph (4.0%), Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph (2.8%), Dung Saprotroph-Undefined Saprotroph (2.3%), Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Soil Saprotroph-Wood Saprotroph (2.2%), Fungal Parasite-Undefined Saprotroph (1.4%); the dominant functional groups of Ecological Area C was Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph (31.4%), Undefined Saprotroph (16.2%), Plant Pathogen (7.6%), Parasite-Undefined Saprotroph (5.2%), unknown (3.6%), Fungal animal Pathogen-Dung Saprotroph-Endophyte-Epiphyte-Plant Saprotroph-Wood Saprotroph (3.3%). There was no significant difference in the number of species in the fungal functional groups of the rhizosphere of cigar tobacco in the different Ecoregions, but the proportion of each functional group was considerably different in the case of fewer Ecoregions.
Figure 4: Variations in the composition of fungal functional groups inferred by FUNGuild.
Bacterial community composition in different eco-logical regions of cigar
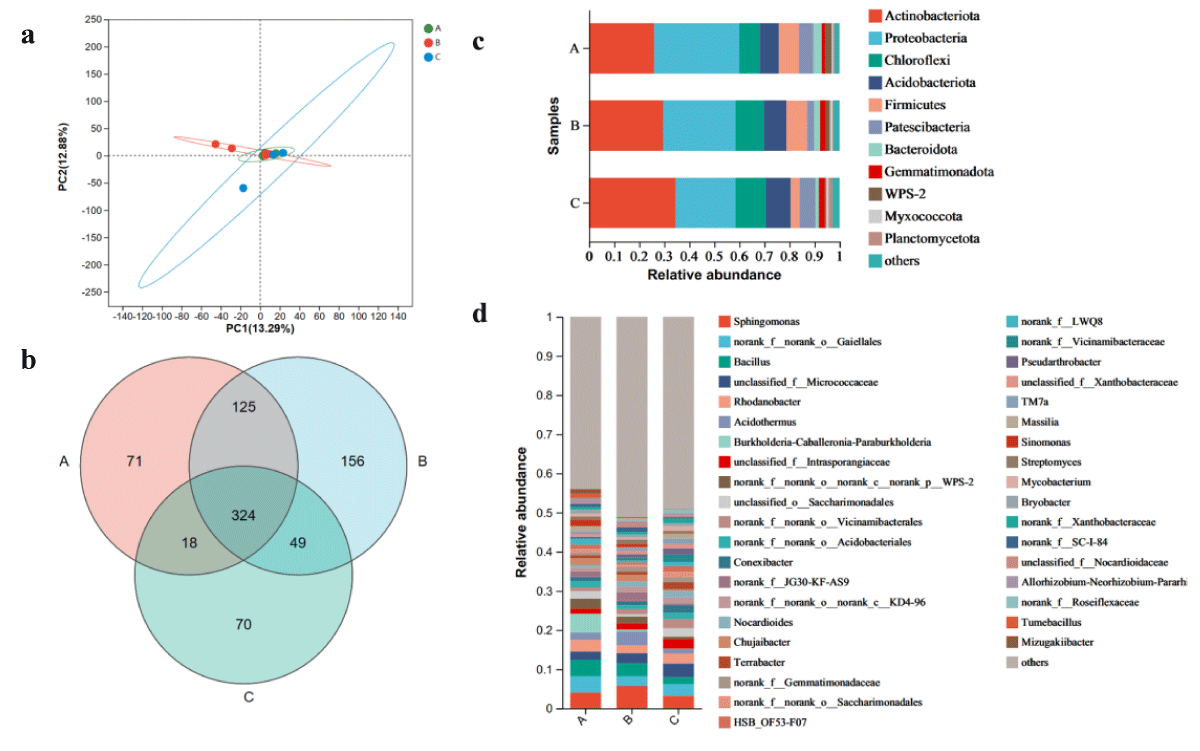
The results of PCoA analysis of rhizospheric soil bacterial communities in EcoregionA, B, and C are shown in Figure 5a.
Figure 5: The microbiome compositions of bacteria taxa in three soil samples. (a) UniFrac-weighted PCoA plots of bacteria communities at phylum level in three samples. (b) Venn diagram of exclusive and shared bacteria species in three soil samples. (c) Bacteria community structure at the level of phylum. (d) The composition of bacteria community at the level of genus. A, Ecology1; B, Ecology2; C, Ecology3; (phyla and genera with average relative abundance (RA) < 1% were merged and indicated as "Others")
The two principal coordinates account for 91.77% of the variability, with PC1 accounting for 13.29% and PC2 accounting for 12.88%. Moreover, the PCoA analysis shows that the bacterial populations of the microbial communities in Ecozones A, B, and C share some similarities.
Metagenomic sequencing of three cigar soil samples revealed 1535 bacterial species belonging to 40 phyla, 119 classes, 264 orders, 417 families, and 813 genera. 538 species were detected in A, 654 species in B, 461 species in Ecozone C, and 324 species were common to all three populations (Figure 5b). Among them, the dominant bacterial phyla in different ecological regions of cigar were Ascomycota, Proteobacteria, Chloroflexi, Acidobacteriota, Firmicutes, Patescibacteria, Bacteroidota, Gemmatimonadota, WPS-2 and other (RA > 1%)( Figure 5c). The total number of these phyla accounted for more than 98.9% of the bacterial group, and the relative abundance of Ascomycota and Proteobacteria was the highest (59.92%). In addition, Ecozone C has the highest proportion of Ascomycota, followed by Ecozone B and finally Ecozone C, but with the opposite proportion of Proteobacteria.
At the genus level, Sphingomonas, norank_f_norank_o_Gaiellales, Bacillus and unclassified_f_Micrococcaceae were the five most abundant bacterial genera (RA > 2%). Mortierella and Saitozyma have the highest proportion in Ecoregion C; the proportions of norank_f_norank_o_Gaiellales, Bacillus, and Burkhola-Caballeronia-Paraburkholderia in Ecoregion A was significantly higher than those in EcoregionB and C. The proportion of Sphingomonas in Ecoregion B is higher than that in Ecoregion A and Ecoregion C. Among the top five dominant genera, the proportion of Ecoregion C was lower than that of Ecoregion A and Ecoregion B. It should be noted that different ecological regions have obvious effects on the microbial community structure of cigar rhizosphere bacteria, which may provide theoretical support for selecting suitable cigar cultivation regions in the future.
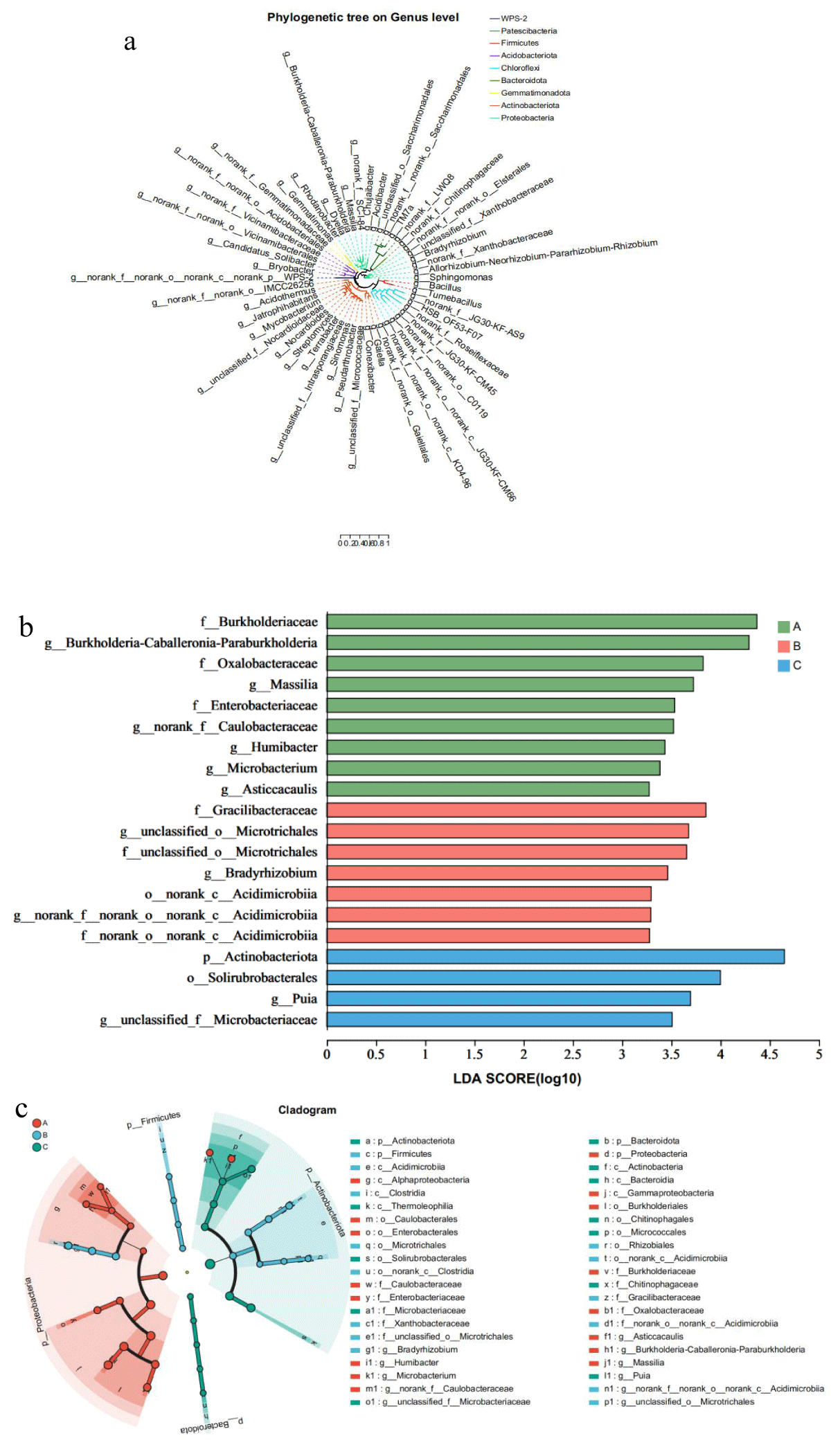
The phylogenetic relationships of the microbes detected by high-throughput sequencing indicated that the sum of the cigar rhizosphere-associated microbial communities originated from various phyla (Figure 6). In order to further study which microorganisms were significantly enriched in the rhizosphere of cigar tobacco, LEfSe analysis was used. The results showed that Proteobacterium was significantly enriched in Ecoregion A; Expectorate and Bacteroidota were significantly enriched in Ecoregion C; Firmicutes were significantly enriched in Region B. In conclusion, the observations presented in this paper suggest that different economies affect the enrichment of Eubacteria in the rhizosphere of cigars.
Figure 6: Comparison of microorganisms and bacteria in the rhizosphere of cigar tobacco in different Ecoregions(a)Cladogram of 50 microbes (mean relative abundance) based on taxonomy. (b) LDA scores of biomarker bacteria for each combination of cigar sites. LDA scores are shown as horizontal bars for the biomarker fungal with an LDA score > 4.8 as listed on the left, Kruskal–Wallis rank sum test, p < 0.05. LDA, linear discriminant analysis. (c) LEfSe analysis revealed distinct taxa of cigar tobacco rhizosphere bacteria in different Ecoregions. Areas of different colors represent different components (red for Ecoregion 1, green for Ecoregion 2, green for Ecoregion 3). The diameter of each circle is proportional to the relative abundance of the taxa, and the inner circle to the outer ring corresponds to the phylum to the genus layer.
The cigar is an economic crop that is easily affected by the ecological environment, and the ecological conditions in different regions can considerably affect or determine the flavor profile of tobacco. Among various environmental factors, previous studies have mainly focused on the effects of climate, soil fertility, altitude, and other factors on cigar growth in different Ecoregions. At present, there are few reports on the effects of rhizosphere soil microorganisms on cigar growth in different ecological regions [20]. This study discussed the effect of ecological region on the distribution of microbial community structure in cigar rhizosphere soil, because microorganism is an essential component of soil, and the soil environment with a large number of microorganisms and abundant diversity is beneficial to the growth of cigar and the stability of soil structure. Increasing the yield of cigars may also significantly affect the synthesis of cigar aroma [21]. In this study, we found that the diversity of bacteria and fungi in cigar rhizosphere soils in different ecological regions exhibit significantly different community structure distributions.
Fungi are one of the leading members of the rhizosphere soil microorganisms, with a wide variety and wide distribution, the ability to adapt to a wide range of adverse conditions, and the ability to decompose a wide variety of organic substances and soil constituents, thus regulating the soil carbon and nitrogen balance [22-25]. However, 70% of diseases in soil are derived from soil fungi [26]. Modifying soil fungal diversity is therefore a particularly promising direction of development with potential applications in improving soil quality and enhancing agroecosystem productivity. Mechanisms of Ecoregion-driven microbial community shift have been an influential line of research. The diversity and community structure of soil fungi shows a variety of patterns as ecological regions alter. In this study, the Simpson, Shannon, Ace, and Chao indices of soil fungi in three different ecological regions were different (Table 1). The Shannon, Simpson, Shannon, Ace, and Chao indices of soil fungi in ecological region C were higher than those in ecological region B and ecological region A, and there were significant differences between ecological region C and ecological region B. The results showed that Ascomycota, Mortierellomycota, Basidiomycota, and unclassified_k_Fungi were the dominant microorganisms in the rhizosphere of cigar tobacco in the three ecological regions. Studies have shown that the majority of Ascomycetes are saprophytic fungi that primarily act as major decomposers of organic matter in soil, with the ability to degrade decaying organic substrates [27]. The relative abundance of Ascomycetes is above 60%, which may be due to its strong asexual reproduction, which may produce a large number of conidia and rapidly increase, making it dominant in numbers. At the genus level, Saitozyma and Penicillium are the dominant genera, with the highest relative abundance in Ecoregion A; Saitozyma is the dominant genus and has the highest relative abundance in Ecoregion B; Mortierella and Saitozyma are the dominant genera in Zone C, with the highest relative abundance. The reason for this may be that the fungal recruitment ability of the rhizosphere soil of the cigar is different in different ecological conditions.
| Table 1: α-diversity of bacterial and fungal communities in rhizosphere soil of healthy and root rot-infected tobacco plants in continuous cropping field. | ||||||
| Bacteria/Fungi | Sample | Simpson | Shannon | Ace | Chao | Coverage |
| Fungi | A | 0.0036 ± 0.0015b | 6.05 ± 0.35a | 627 ± 37.64a | 627 ± 37.64a | 1 |
| B | 0.0037 ± 0.0004b | 5.53 ± 0.23b | 542.5 ± 37.63b | 542.5 ± 37.63b | 1 | |
| C | 0.0064 ± 0.0001a | 6.12 ± 0.08a | 631 ± 32.91a | 631 ± 32.91a | 1 | |
| Bacteria | A | 0.0771 ± 0.0094a | 4.50 ± 0.48a | 503.67 ± 7.37a | 503.67 ± 7.37a | 1 |
| B | 0.0539 ± 0.0026b | 4.05 ± 0.26a | 408.25 ± 47.14b | 408.25 ± 47.14b | 1 | |
| C | 0.0237 ± 0.0044c | 3.39 ± 0.06b | 387 ± 38.15b | 387 ± 38.15b | 1 | |
Bacteria are an influential component of soil microorganisms and play an essential role in promoting the soil nutrient cycle, organic matter formation and decomposition, fertility maintenance, plant nutrient absorption, and growth and development. Its diversity and community composition structure is affected by environmental factors, soil characteristics, plant species, and different planting methods [28-30]. Ecoregions affect crop distribution and bacterial community diversity in crop rhizosphere soils through various factors. Simpson, Shannon, Ace, and Chao showed that the bacterial community index in cigar rhizosphere soils varies across ecological regions, as does the richness and diversity of bacterial communities. The Simpson, Shannon, Ace, and Chao indices are higher in Ecoregion A than in Ecoregion B and C, with significant differences. The diversity of rhizosphere soil bacteria in different ecological regions may be different because the different environmental factors such as soil temperature and humidity in ecological regions alter the soil micro-ecological environment for crop growth, such as the difference of root exudates, agro-chemicals, and other substances affect the microbial activities in rhizosphere soil, thus leading to the difference of bacterial community structure and diversity in different ecological regions [31,32]. In this study, the dominant microbial phyla in the three ecological regions were Ascomycota and Proteobacteria (≧ 59.92%), but the relative abundance of the dominant bacterial phyla in each ecological region was different. Among them, the relative abundance of the dominant bacterial phylum Ascomycota is as follows: C>B>A and the relative abundance of Proteobacteria was in the order of A>B>C. At the genus level, the dominant genera were Other, Sphingomonas, norank_f_norank_o_Gaiellales, Bacillus, and unclassified_f_Micrococcaceae (RA > 2%). The dominant genera with the highest relative abundance in Ecological Area A is Burkholderia-Caballeronia-Paraburkholderia; Sphingomonas is the dominant genus with the highest relative abundance in Ecological Area B. The dominant genus with the highest relative abundance in the Ecological Area C region is Norank_f_norank_o_Gaiellales. The results suggest that different Ecoregions may affect the distribution and diversity of bacterial communities in cigar rhizosphere soils.
Relevant studies have shown that tobacco quality is the result of the joint action of genetic factors, ecological factors, and cultivation techniques, and the synergy of the three is the basis for producing high-quality tobacco leaves [33,34]. Genetic factors are the internal causes, and cultivation techniques can be artificially adjusted and controlled according to different ecological conditions and varieties. While some factors can be modified to some extent through science and technology, in most cases it is difficult to modify ecological factors. Due to the relative stability of the ecological environment, the style and quality of tobacco leaves produced in different ecological regions have obvious and irreplaceable regional characteristics and ecological advantages [35-37]. According to relevant studies, the contribution rates of ecological environment, planting varieties, and cultivation techniques to tobacco quality are about 58%, 32% and 10% respectively [38-40]. It can be seen that ecological conditions are the most influential and basic factors determining the quality of tobacco leaves, and are the basis and premise for the formation of excellent quality [41,42]. However, the deficiency of this study is that we only focus on the effect of different ecological zones on the structure of the microbial community in the soil of the cigar rhizosphere and do not incorporate a multi-factor analysis of cultivation techniques, cultivars, and cigar quality. In the following, we examine the effect of different ecological zones, cultivation techniques, and cultivars on the microbial community structure of cigar tobacco rhizosphere soils. The mass of cigar tobacco was analyzed to explore the mass formation mechanism of cigar tobacco. Different cultivation techniques, ecological environments, and varieties grown in different production areas are completely considered to determine the optimal cultivation techniques for each production area.
In this study, the effect of different ecological environments on the structure of the fungal and bacterial communities in the rhizosphere of cigar tobacco at maturity was investigated using high-throughput sequencing. The results showed that: i) The diversity of the fungal community in the rhizosphere soil of cigar tobacco in ecological region C was higher than that in ecological region B and ecological region A, while the diversity of the bacterial community in ecological region A was higher than that in ecological region B and ecological region C; ii) The dominant fungi in the rhizosphere soil of cigar tobacco in three different ecological regions mainly included Ascomycota, Mortierellomycota and Basidiomycota, but the dominant fungi genera were different in different ecological regions; the dominant microbial in the rhizosphere of cigar tobacco in the three different ecological zones are mainly Ascomycota and Proteobacteria, and the dominant microbial in the rhizosphere are mainly Othere, Sphingomonas, norank_f__norank_o__Gaiellales, Bacillus and unclassified_f__Micrococcaceae; iii) Different ecological environments affected the fungal and bacterial communities in the rhizosphere soil of cigar tobacco.
Funding
This work was supported by a Major special research project of Yunnan Tobacco Company "Research and application of key technology of Cigar Tobacco leaf homogenization production"(2022530000241005).
- Yang XY, Jin DM, Song SX. Adaptability evaluation of introduced cigar tobacco varieties in Sichuan Wanyuan tobacco-growing area. Crop Research. 2018; 32(6):504-540.
- Zhang J W. GIS-based ecological suitability assessment of main cigar growing areas in Sichuan Province. Henan Agricultural University. 2021.
- Deng YG. Time to the east. Research progress on ecological adaptability and cultivation techniques of cigar-tomato varieties. Journal of Jiangxi Agriculture. 2021; 33 (01):60-66.
- Ma GM, Tan T, Yang D. Study on pathogen the identification of cigar root-knot nematode disease and control effect of biogenic ammonia fumigation in Jiangcheng, Yunnan. Journal of Yunnan University (Natural Science Edition). 2023;45 (01): 211-217.
- Lin BS, Gao HJ, Zheng Q, Cai B. Comparative study on climatic conditions of main cigar producing areas at home and abroad. Journal of Tropical Crops. 2022; 1:15.
- Qin YQ, Li AJ, Fan JY. Discussion on production technology of high-quality cigar coat. Journal of Jiangxi Agriculture. 2012; 07:101-103.
- Wang HY, Zuo XJ, Sun FS. Research progress of cigar wrapper. China Tobacco Science. 2009; 05:71-76.
- Tao J, Liu HB, Xin YH. Characteristics of main ecological factors in high-quality cigar growing area of Pinar del Rio Province, Cuba. Chinese Journal of Tobacco. 2016; 22 (04):62-69.
- Li AJ, Qin YQ, Fan JY. Advantage analysis and countermeasures of developing cigar tobacco production in Sichuan Province. Anhui Agricultural Sciences. 2013; (07): 3188-3189, 3228.
- Gao WK, Li MS, Li C. Analysis of fungal community structure and ecological characteristics in rhizosphere soil of flue-cured tobacco at different altitudes. Jiangsu Agricultural Science. 2022; 50 (08):221-227.
- Zhou YJ, Jia X, Zhao YH. Altitudinal distribution pattern of fungal community in Huoditang, Qinling Mountains. Chinese Journal of Applied Ecology. 2021; 32 (7):2589-2596.
- Liu Y. Study on the Difference of Soil Microbial Community in Paddy Field in Different Ecological Regions and the Mechanism of Deep Tillage. Henan Agricultural University. 2022.
- Zhuo LX. Diversity analysis of soil bacteria and nitrogen-fixing microorganisms in different ecological regions of Shaanxi. Northwestern University. 2017.
- Yuan YW. Study on soil fertility status of tobacco-growing at different altitudes in Zhangjiajie tobacco-growing area. Changsha: Hunan Agricultural University. 2014; 40-42.
- Kyselková M, Kopecký J, Frapolli M, Défago G, Ságová-Marecková M, Grundmann GL, Moënne-Loccoz Y. Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J. 2009 Oct;3(10):1127-38. doi: 10.1038/ismej.2009.61. Epub 2009 Jun 25. PMID: 19554036.
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016 Jul;13(7):581-3. doi: 10.1038/nmeth.3869. Epub 2016 May 23. PMID: 27214047; PMCID: PMC4927377.
- Louca S, Parfrey LW, Doebeli M. Decoupling function and taxonomy in the global ocean microbiome. Science. 2016 Sep 16;353(6305):1272-7. doi: 10.1126/science.aaf4507. PMID: 27634532.
- Chu H, Gao GF, Ma Y, Fan K, Delgado-Baquerizo M. Soil Microbial Biogeography in a Changing World: Recent Advances and Future Perspectives. mSystems. 2020 Apr 21;5(2):e00803-19. doi: 10.1128/mSystems.00803-19. PMID: 32317392; PMCID: PMC7174637.
- Zhou Z H. Identification of main ecological factors and suitable interval extraction for quality difference of tobacco leaves in Liangpan tobacco-growing areas. Chengdu. Journal of Sichuan Agricultural University. 2017.
- He HB, Li WX, Zhang YW. Effects of Italian ryegrass residues as green manure on soil properties and bacterial communities under an Italian ryegrass(Lolium multiflorum L.)rice(Oryza sativa L.)rotation. Soil and tillage research. 2020; 196:104487.
- Merckx VSFT, Gomes SIF, Wapstra M. The biogeographical history of the interaction between mycoheterotrophic Thismia (Thismiaceae) plants and mycorrhizal Rhizophagus (Glomeraceae) fungi. Journal of Biogeography. 2017; 44(8): 1869-1879.
- Ma Q, Xia DS, Wu ZH. Diversity analysis of soil fungi in western Inner Mongolia. Science Technology and Engineering. 2020; 20(35): 14447-14454.
- Frᶏc M, Hannula S E, Bełka M, et al. Frąc M, Hannula SE, Bełka M, Jędryczka M. Fungal Biodiversity and Their Role in Soil Health. Front Microbiol. 2018 Apr 13;9:707. doi: 10.3389/fmicb.2018.00707. PMID: 29755421; PMCID: PMC5932366.
- Žifčáková L, Větrovský T, Howe A, Baldrian P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol. 2016 Jan;18(1):288-301. doi: 10.1111/1462-2920.13026. Epub 2015 Oct 14. PMID: 26286355.
- Liu Z, Xu YP, Wang XL. Analysis of fungal community structure and diversity in rhizosphere soil of alfalfa in Heilonggang. Jiangsu Agricultural Science. 2021; 49(10): 197-201.
- Labandeira CC, Wappler T. Arthropod and Pathogen Damage on Fossil and Modern Plants: Exploring the Origins and Evolution of Herbivory on Land. Annu Rev Entomol. 2023 Jan 23;68:341-361. doi: 10.1146/annurev-ento-120120-102849. PMID: 36689301.
- Liu C, Cai Q, Liao P. Effects of Fallopia multiflora–Andrographis paniculata intercropping model on yield, quality, soil nutrition and rhizosphere microorganisms of F. multiflora. Plant and Soil. 2021; 467: 465-481.
- Qiu Jie, Hou Yiling, Xu Lili. High-throughput sequencing analysis of bacterial diversity in rhizosphere soil of different mulberry trees. Southern Agricultural Journal. 2019; 50(3):585-592.
- Xi K, Liu M, Wang M. Effects of Seasonal Grazing on Microbial Community Structure in Rhizosphere Soil of Stellera chamaejasme in Gannan Alpine Meadow. International Journal of Social Science and Education Research. 2023; 6(6): 362-372.
- Revilla P, Anibas CM, Tracy WF. Sweet Corn Research around the World 2015–2020. Agronomy. 2021; (11):534.
- Kang LY, Liu ZB, Ou LJ. Effects of capsicum planting on microbial diversity in rhizosphere soil. Journal of Hunan Agricultural University (Natural Science Edition). 2018; 44(2): 151-156, 175.
- Zhu AQ. Effect of ecological factors on quality of flue-cured tobacco in Yuxi area. Nanjing University of Information Science and Technology. 2021.
- Tang YJ, Zhang JP. Preliminary classification of quality ecological types of main flue-cured tobacco production bases in Shanghai. China Tobacco Science. 2006; 27(3): 1-5.
- Yang C. Characteristics of main ecological factors and their effects on yield and quality of flue-cured tobacco in Chongqing tobacco-growing areas. Southwest University. 2015.
- Zhu H, Liang M, Song WJ. Effects of climatic factors on quality and style characteristics of flue-cured tobacco. Jiangsu Agricultural Sciences. 2017; 45(5): 63-69.
- Ju YQ, Chen ZH, Ma D. Analysis on climate the similarity of high-quality flue-cured tobacco growing areas in central and northern subtropics of China. Chinese Journal of Applied Meteorology. 2022; 33 (06):736-747.
- Miki LWX, Li WC. Effects of different light intensity on photosynthetic physiology and biochemistry of two flue-cured tobacco seedlings. Molecular Plant Breeding. 2023; (1): 17.
- Guo JH, Shen JP, Zhang Shixiang. Similarity analysis of physical properties between imported flue-cured tobacco and flue-cured tobacco grown in China. China Tobacco Science. 2018; 39 (01): 91-96.
- Zhao JL, Wang XG, Zhang XLi. Study on ecological adaptability of new flue-cured tobacco varieties in western Henan Province. Hunan Agricultural Sciences. 2021; (03): 6-10.
- Wang J, Li JZ, Ji GF. Effects of ecological deep planting techniques on growth, yield and quality of flue-cured tobacco in Lingbao tobacco-growing area. Anhui Agricultural Science. 2022; 50 (13): 30-37.
- Zhong JH. Effects of different soil and fertilizer application on yield and quality of flue-cured tobacco. Journal of Jiangxi Agriculture. 2019; 31 (04): 69-76.
- Yan J, Shi RY, Wang CJ. Effects of different amendments on acid tobacco fields and growth of flue-cured tobacco. Soils. 2023; 55 (03): 612-618.