More Information
Submitted: June 11, 2022 | Approved: June 17, 2022 | Published: June 20, 2022
How to cite this article: Bel Y, Galeano M, Baños-Salmeron M. Escriche B. The use of Bacillus thuringiensis to control plant-parasitic nematodes. J Plant Sci Phytopathol. 2022; 6: 062-064.
DOI: 10.29328/journal.jpsp.1001076
Copyright License: © 2022 Bel Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The use of Bacillus thuringiensis to control plant-parasitic nematodes
Yolanda Bel1, Magda Galeano2, Mireya Baños-Salmeron2 and Baltasar Escriche1*
1BIOTECMED Institute, Department of Genetics, University of Valencia, Spain
2Koppert España, SL, Almeria, Spain
*Address for Correspondence: Dr. Baltasar Escriche, BIOTECMED Institute, Department of Genetics, University of Valencia, Dr. Moliner, 50. 46100-Burjassot, Valencia, Spain, Email: [email protected]
Plant-parasitic nematodes are ubiquitous in nature and cause large losses in agriculture. The current concerns regarding the use of chemical pesticides have increased the interest in new control alternatives. One of these is the one based on Bacillus thuringiensis (Bt). These Gram-positive bacteria have the ability to synthesize pesticide proteins during sporulation. Some of these proteins have nematicidal properties. Studies have shown that preparations of certain strains of Bt can prevent or slow down the infestation of phytonematodes. The expression of some Bt nematicidal genes in transgenic plants has also demonstrated their effectiveness. Bt is nowadays an effective ecological alternative for controlling plant-parasitic nematodes.
Nematodes are the dominant fauna in most soil commu-nities [1]. There are more than 4100 species of plant-parasitic nematodes, which cause severe damage to crops all around the world. The magnitude of the losses depends fundamentally on population densities in soil and roots, susceptibility of the crop, and environmental conditions such as the temperature of the soil, which largely affect the development of nematodes. Some plant-parasitic nematodes cause severe crop losses to food and fiber crops [2], annually estimated to be US $78 billion worldwide [3]. In the Mediterranean area, production losses by the root-knot nematodes on the horticultural crops have been estimated to be between 15 and 60% [4].
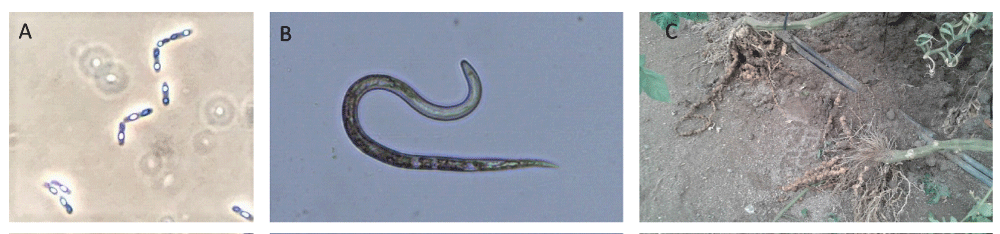
Plant-parasitic nematodes are hidden and unseen enemies of crop plants as they are microscopic and are present in the soil. They can be grouped as (A) Sedentary endoparasites, the cyst and root-knot nematodes, the most damaging obligate plant-endoparasitic nematodes affecting a wide range of plant species worldwide (Figure 1) [5]; (B) Semi sedentary endoparasites, (C) Migratory endoparasites (D) Virus transmitter nematodes, and (E) Stem and leaf damaging nematodes. The plant-parasitic nematodes that are considered to cause the greatest social and economic impacts are: root-knot nematodes (Meloidogyne spp.), cyst nematodes (Heterodera and Globodera spp.), root-lesion nematodes (Pratylenchus spp.), the burrowing nematode Radopholus similis, the migratory endoparasite Ditylenchus dipsaci, the pine wilt nematode Bursaphelenchus xylophilus, the reniform nematode Rotylenchulus reniformis, the virus vector nematode Xiphinema index, the false root-knot Nacobbus aberrans, and Aphelenchoides besseyi, an important pathogen of rice [5]. In general, nematode infection results in above and ground symptoms in plants, like general plant wilting, leaf necrosis, chlorosis, leaf dropping, stunted growth, and in the case of penetration to the root systems, root gall and knot formation. Furthermore, nematicidal infection results in enhanced susceptibility to other pathogens [6].
Figure 1: Bt and root-knot nematode. Bacillus thuringiensis spores and crystals (A), J2 stage of Meloidogyne javanica (B), and tomato roots affecte by root-knot nematodes (C).
The necessity of increasing the food (crop) production by at least 2% every year to assure an appropriate world food supply [7] drives the search for agricultural protection systems against pests while preserving the environment and avoiding the accumulation of chemical residues in nature. Bacillus thuringiensis (Bt), is a well-known entomopathogen. It is a Gram-positive ubiquitous bacterium distributed worldwide. One of its most known characteristics is that during sporulation it produces parasporal crystals composed of pesticide proteins (Figure 1) [8,9]. This bacterium is safe for other organisms, including humans [10]. Therefore, Bt-based compounds have been used as the most successful microbial insecticides for decades [11,12] and it is one of the most promising biological control agents.
The pesticide proteins produced by Bt are toxic to larvae of many insect species and also to nematodes [9,13,14]. To exert its toxic action, it is widely accepted that the Bt pesticidal proteins included in the crystalline inclusion bodies have to be solubilized in the midgut of the susceptible organism. After activation and binding to specific midgut membrane receptors, several processes can happen, such as the activation of intracellular death pathways [15]. Additionally, or alternatively, the sequential biding model processes can take place: oligomer promotion, insertion in the membrane, and pore formation that break the epithelial cells and also allow the bacteria to infect hemocoel causing septicemia [16,17].
The toxicity of Bt to nematodes is well established since 1972 when the first study showing the toxicity of Bt against Meloidogyne spp. was published [18]. Seven classes of Bt Cry toxins have been reported to have activity against nematodes: Cry5, Cry6, Cry12, Cry13, Cry14, Cry21, and Cry55 [14,19,20]. In addition, other Bt proteins apart from Cry (such as thuringiensin, chitinase, and metalloproteinases) are toxic to nematodes (as a review, see [19,21]) which can increase its effectivity. As an additional beneficial effect, Bt can also promote plant growth [22-24]. The mode of action of Bt Cry toxins in nematodes is not well established, but it is known that carbohydrates are essential for Cry5B toxicity to Caenorhabditis elegans, to allow binding and therefore toxicity [25], that cadherin acts as a receptor [26] and that Bt also targets the intestinal epithelial junctions in this organism [27].
The effectivity of Bt in controlling plant-parasitic nematodes such as Meloidogyne hapla has been reported, after soil drenching with spore-crystal mixtures of Cry6 in tomato plants, decreasing galling index and egg masses on host root, and reducing the final number of nematodes in soil [28]. Similar results have been obtained against Meloidogyne incognita after treating tomato plants with Bt strains, which were also able to translocate into the plant tissues [29]. The susceptibility of M. incognita to Cry5, Cry6, and Cry55 proteins is well established [30,31]. Additionally, it has been published that Meloidogyne javanica infestation was reduced after Bt treatment [32].
Apart from the conventional use of Bt for crop treatments, transgenic Bt crops expressing Lepidopteran and Coleopteran active proteins have been developed and commercialized since 1996. The Bt crops can control successfully the target pests and are planted in several countries in the world since the last years of the past century ([33], https://www.isaaa.org/gmapprovaldatabase/default.asp, last accessed 2nd June 2022). Similarly, the gene coding for Cry5B protein has been transformed in tomato plants and in the fungus Botrytis cinerea to control M. incognita and the pine wood nematode Bursaphelenchus xylophilus respectively [34,35] with successful results in the control of the two phytonematodes. Also, it has been reported that tomato roots expressing Cry6A decreased M. incognita population [36] and soybean transformed with the Cry14 gene showed a reduction of soybean cyst nematode Heterodera glycines adults and eggs [37]. Indeed, the US Environmental Protection Agency recently approved the registration of transgenic soybean GMB151 targeting the soybean cyst nematode (https://www.isaaa.org/gmapprovaldatabase/event/default.asp?EventID=562, last accessed 2nd June 2022).
Summarizing, Bt strains or Bt Cry proteins can be excellent nematicidal agents that can be part of new generation strategies for the control of plant-parasitic nematodes.
We thank the European Union’s Horizon 2020 Research and Innovation program, under Grant Agreement no. 773554 (EcoStack Project) for their support.
- Marahatta SP, Wang KH, Sipes BS, Hooks CR. Effects of the integration of sunn hemp and soil solarization on plant-parasitic and free-living nematodes. J Nematol. 2012 Mar;44(1):72-9. PMID: 23482700; PMCID: PMC3593259.
- Stirling GR. Biological control of plant-parasitic nematodes: an ecological perspective, a review of progress and opportunities for further research. Davies K, Spiegel Y, editors. Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms. 2011; 1-38.
- Barker KR, Pederson GA, Windham GL. Plant and Nematode Interactions. American Society of Agronomy. 1998.
- Andres MF, Verdejo-Lucas S. Enfermedades causadas por nematdos fitoparásitos en España. Phytoma. 2011.
- Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, Perry RN. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013 Dec;14(9):946-61. doi: 10.1111/mpp.12057. Epub 2013 Jul 1. PMID: 23809086; PMCID: PMC6638764.
- Ali MA, Azeem F, Abbas A, Joyia FA, Li H, Dababat AA. Transgenic Strategies for Enhancement of Nematode Resistance in Plants. Front Plant Sci. 2017 May 9;8:750. doi: 10.3389/fpls.2017.00750. PMID: 28536595; PMCID: PMC5422515.
- Sikora R, Molendijk LPG, Desaeger J. Integrated nematode management and crop health: future chalenges and opportunuties. CABI. 2021; 3-10.
- van Frankenhuyzen K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J Invertebr Pathol. 2009 Apr;101(1):1-16. doi: 10.1016/j.jip.2009.02.009. Epub 2009 Mar 6. PMID: 19269294.
- Palma L, Muñoz D, Berry C, Murillo J, Caballero P. Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins (Basel). 2014 Dec 11;6(12):3296-325. doi: 10.3390/toxins6123296. PMID: 25514092; PMCID: PMC4280536.
- Raymond B, Federici BA. In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity-a response to EFSA. FEMS Microbiol. Ecol. 2017; 93(7).
- Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotechnol J. 2011 Apr;9(3):283-300. doi: 10.1111/j.1467-7652.2011.00595.x. Epub 2011 Feb 25. PMID: 21375687.
- Sanchis V. From microbial sprays to insect-resistant transgenic plants: history of the biospesticide Bacillus thuringiensis. A review. Agron Sustain Dev. 2011; 31(1):217-231.
- Salehi Jouzani G, Seifinejad A, Saeedizadeh A, Nazarian A, Yousefloo M, Soheilivand S, Mousivand M, Jahangiri R, Yazdani M, Amiri RM, Akbari S. Molecular detection of nematicidal crystalliferous Bacillus thuringiensis strains of Iran and evaluation of their toxicity on free-living and plant-parasitic nematodes. Can J Microbiol. 2008 Oct;54(10):812-22. doi: 10.1139/w08-074. PMID: 18923549.
- Jouzani GS, Valijanian E, Sharafi R. Bacillus thuringiensis: a successful insecticide with new environmental features and tidings. Appl Microbiol Biotechnol. 2017 Apr;101(7):2691-2711. doi: 10.1007/s00253-017-8175-y. Epub 2017 Feb 24. PMID: 28235989.
- Ibrahim MA, Griko N, Junker M, Bulla LA. Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng Bugs. 2010 Jan-Feb;1(1):31-50. doi: 10.4161/bbug.1.1.10519. PMID: 21327125; PMCID: PMC3035146.
- Adang MJ, Crickmore N, Jurat-Fuentes JL. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. In: Advances in Insect Physiology. Dhadialla TS, Gill SS, editors. Oxford: Academic Press. 2014; 39-87.
- Pardo-López L, Soberón M, Bravo A. Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev. 2013 Jan;37(1):3-22. doi: 10.1111/j.1574-6976.2012.00341.x. Epub 2012 Jun 11. PMID: 22540421.
- Prasad SSSV, Tilak K, Gollakota K. Role of Bacillus thuringiensis var. thuringiensis on the larval survivability and egg hatching of Meloidogyne spp., the causative agent of root knot disease. J Invertebr Pathol. 1972; 20:377-378.
- Ruan L, Crickmore N, Peng D, Sun M. Are nematodes a missing link in the confounded ecology of the entomopathogen Bacillus thuringiensis? Trends Microbiol. 2015 Jun;23(6):341-6. doi: 10.1016/j.tim.2015.02.011. Epub 2015 Mar 25. PMID: 25818004.
- Wei JZ, Hale K, Carta L, Platzer E, Wong C, Fang SC, Aroian RV. Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2760-5. doi: 10.1073/pnas.0538072100. Epub 2003 Feb 21. PMID: 12598644; PMCID: PMC151414.
- Horak I, Engelbrecht G, van Rensburg PJJ, Claassens S. Microbial metabolomics: essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J Appl Microbiol. 2019 Aug;127(2):326-343. doi: 10.1111/jam.14218. Epub 2019 May 23. PMID: 30739384.
- Radhakrishnan R, Hashem A, Abd Allah EF. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front Physiol. 2017 Sep 6;8:667. doi: 10.3389/fphys.2017.00667. PMID: 28932199; PMCID: PMC5592640.
- Azizoglu U. Bacillus thuringiensis as a Biofertilizer and Biostimulator: a Mini-Review of the Little-Known Plant Growth-Promoting Properties of Bt. Curr Microbiol. 2019 Nov;76(11):1379-1385. doi: 10.1007/s00284-019-01705-9. Epub 2019 May 17. PMID: 31101973.
- Tomita Y, Yamazaki K, Aiuchi D, Asano S-i, Koike M. Plant disease control and PGPR effects by entomopathigenic Bacillus thuringiensis. In: IOBC-WPRS Bulletin-Microbial and Nematode Control of Invertebrate Pests, IOBC-WPRS. 1502020. 170-173.
- Griffitts JS, Whitacre JL, Stevens DE, Aroian RV. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science. 2001 Aug 3;293(5531):860-4. doi: 10.1126/science.1062441. PMID: 11486087.
- Peng D, Wan D, Cheng C, Ye X, Sun M. Nematode-specific cadherin CDH-8 acts as a receptor for Cry5B toxin in Caenorhabditis elegans. Appl Microbiol Biotechnol. 2018 Apr;102(8):3663-3673. doi: 10.1007/s00253-018-8868-x. Epub 2018 Mar 3. PMID: 29502179.
- Wan L, Lin J, Du H, Zhang Y, Bravo A, Soberón M, Sun M, Peng D. Bacillus thuringiensis targets the host intestinal epithelial junctions for successful infection of Caenorhabditis elegans. Environ Microbiol. 2019 Mar;21(3):1086-1098. doi: 10.1111/1462-2920.14528. Epub 2019 Feb 14. PMID: 30637902.
- Yu Z, Xiong J, Zhou Q, Luo H, Hu S, Xia L, Sun M, Li L, Yu Z. The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J Invertebr Pathol. 2015 Feb;125:73-80. doi: 10.1016/j.jip.2014.12.011. Epub 2014 Dec 31. PMID: 25556591.
- Verduzco-Rosas LA, García-Suárez R, López-Tlacomulco JJ, Ibarra JE. Selection and characterization of two Bacillus thuringiensis strains showing nematicidal activity against Caenorhabditis elegans and Meloidogyne incognita. FEMS Microbiol Lett. 2021 Apr 8;368(5):fnaa186. doi: 10.1093/femsle/fnaa186. PMID: 33720297.
- Geng C, Liu Y, Li M, Tang Z, Muhammad S, Zheng J, Wan D, Peng D, Ruan L, Sun M. Dissimilar Crystal Proteins Cry5Ca1 and Cry5Da1 Synergistically Act against Meloidogyne incognita and Delay Cry5Ba-Based Nematode Resistance. Appl Environ Microbiol. 2017 Aug 31;83(18):e03505-16. doi: 10.1128/AEM.03505-16. PMID: 28710264; PMCID: PMC5583498.
- Peng D, Chai L, Wang F, Zhang F, Ruan L, Sun M. Synergistic activity between Bacillus thuringiensis Cry6Aa and Cry55Aa toxins against Meloidogyne incognita. Microb Biotechnol. 2011 Nov;4(6):794-8. doi: 10.1111/j.1751-7915.2011.00295.x. Epub 2011 Sep 19. PMID: 21923640; PMCID: PMC3815414.
- Khyami-Horani H, Al-Banna L. Efficacy of Bacillus thuringiensis jordanica against Meloidogyne javanica infecting tomato. Phytopathol. Mediterr. 2006; 45(2):153-157.
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change. ISAAA Brief No. 54. ISAAA: Ithaca, NY. 2018.
- Li X-Q, Tan A, Voegtline M, Bekele S, Chen C-S, Aroian RV. Expression of Cry5B protein from Bacillus thuringiensis in plant roots confers resistance to root-knot nematode. Biol. Control. 2008; 47(1):97-102.
- Cheng C, Qin J, Wu C, Lei M, Wang Y, Zhang L. Suppressing a plant-parasitic nematode with fungivorous behavior by fungal transformation of a Bt cry gene. Microb Cell Fact. 2018 Jul 23;17(1):116. doi: 10.1186/s12934-018-0960-5. PMID: 30037328; PMCID: PMC6055344.
- Li XQ, Wei JZ, Tan A, Aroian RV. Resistance to root-knot nematode in tomato roots expressing a nematicidal Bacillus thuringiensis crystal protein. Plant Biotechnol J. 2007 Jul;5(4):455-64. doi: 10.1111/j.1467-7652.2007.00257.x. Epub 2007 Apr 19. PMID: 17451491.
- Kahn TW, Duck NB, McCarville MT, Schouten LC, Schweri K, Zaitseva J, Daum J. A Bacillus thuringiensis Cry protein controls soybean cyst nematode in transgenic soybean plants. Nat Commun. 2021 Jun 7;12(1):3380. doi: 10.1038/s41467-021-23743-3. PMID: 34099714; PMCID: PMC8184815.