More Information
Submitted: February 10, 2022 | Approved: March 03, 2022 | Published: March 04, 2022
How to cite this article: Garg AN, Singh R, Maharia RS, Dutta RK, Datta A. Quantification of minor, trace and toxic elements in stems of Santalum album (L.), Mangiferra indica (L.) and Tinospora cordifolia by instrumental neutron activation analysis. J Plant Sci Phytopathol. 2022; 6: 008-014.
DOI: 10.29328/journal.jpsp.1001067
Copyright License: © 2022 Garg AN, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Elemental analysis; INAA; Micronutrients; Santalum album; Mangiferra indica; Tinospora cordifolia; Ca content
Quantification of minor, trace and toxic elements in stems of Santalum album (L.), Mangiferra indica (L.) and Tinospora cordifolia by instrumental neutron activation analysis
Garg AN1* , Ruchi Singh2, Maharia RS1, Dutta RK1 and Arpita Datta3
, Ruchi Singh2, Maharia RS1, Dutta RK1 and Arpita Datta3
1Department of Chemistry, Indian Institute of Technology, Roorkee 247667, India
2Dev Sanskriti Univesity, Gayatri Kunj, Haridwar 249411, India
3Institute of Nuclear Science & Technology, Amity University, Noida 201313, India
*Address for Correspondence: Prof. Garg AN, Retired Professor of Chemistry, Indian Institute of Technology, Roorkee 247667, India, Email: [email protected]
Stems of Santalum album (Sandalwood), Mangiferra indica (Mango wood), and Tinospora cordifolia (Giloy) are widely used in the preparation of herbal medicines and formulations in the traditional Indian health care system called Ayurveda. These were analyzed for 4 minor (K, Ca, Cl, Mg) and 13 traces (As, Ce, Co, Cr, Cu, Fe, Hg, La, Mn, Na, Se, V, and Zn) including toxic elements by instrumental neutron activation analysis (INAA). Samples in powder form along with reference materials (NIST SRM 1547 and INCT MPH-2) as comparators were irradiated for 1 min/6 h in Dhruva/CIRUS reactors at BARC, Mumbai. Gamma activity was measured by high-resolution gamma-ray spectrometry. In general, K, Ca, Fe, Mn, and Zn contents are very high in all the samples but Santalum album, widely used as a perfume, is more enriched in K, Ca, Cr, Zn, and Se. The concentration of Ca is always high as a major constituent (> 10 mg/g) in all the stem/bark of plant species. A strong inverse correlation (R2 = 0.9999) was observed between Fe and Zn in all three samples and that may be useful in drug manufacturing.
Many civilizations around the world have extensively used the plant kingdom as the source of herbal medicines. Santalum album L. (Sandalwood) (Figure 1A), Mangiferra indica L. (mango wood) (Figure 1B), and stems of Tinospora cordifolia (giloy) (Figure 1C) are essential ingredients of many herbal formulations used in the traditional Indian health care system called Ayurveda known since 2500 BC. Sandalwood and mango wood are also used in yagya (holy fire) and sandalwood is widely used to spread fragrance [1]. All these plants have been known for ages and are widely found in India, Sri Lanka, and Myanmar. Sandalwood is primarily used as a coolant and its oil is prepared by steam distillation with an average content of 4.5% - 2.5%. It is extensively used in perfumes, cosmetics, and its aqueous paste is a sacred unguent. It exhibits significant antipyretic effects, sedative effects, and astringent activity, making it useful as a disinfectant in genitourinary and bronchial tracts, diuretic expectorant, and stimulant. Essential oil of S album contains more than 90% sesquiterpene alcohols of which 50% - 60% is the tricyclic α-santalol and 20% - 25% is β-santalol [2]. Also, it has been tested for in vitro activity against the Herpes simplex virus [3]. Sandalwood is commonly known as Chandana in India and its powder has antiseptic properties that prevent pimples, acne, sores and reduce wrinkles. Aqueous extract of mango wood stem bark is proposed as a nutritional supplement (antioxidant) and used as an anti-inflammatory, analgesic, and immunomodulatory including dermatological disorders [4].
Figure 1: Typical pictures of stem pieces of (A) Santalum album, (B) Mangiferra indica, (C) Tinospora cordifolia.
Giloy (T cordifolia) or Guduchi is a glabrous, woody climbing shrub belonging to the family of Menispermaceae. Though all its parts are useful stem is particularly used in the treatment of fever, jaundice, emaciation, urinary problem, diabetes, skin diseases, heart disease, diarrhea and dysentery, leprosy, and helminthiasis [5]. Sharma, et al. [6] have reviewed its diverse pharmacological importance in terms of its chemical constituents such as glycosides, alkaloids, steroids, diterpenoid lactones, sesquiterpenoid, and lignans that establish the phytochemistry and pharmacological activity. It exhibits antifungal activity, antioxidant activity, antiallergic, antiviral, antimicrobial, and antibacterial activity, hypolipidemic effect, anti-HIV potential, antitoxic effect, and wound healing activity. Kapil and Sharma [7] have found T cordifolia to possess immune potentiating compounds exhibiting anti-complementary and immunomodulatory activities. Recently Khan and Rathi [8] have reviewed attempts of T cordifolia being used for enhancing the immune system, prevention, management, and development of new therapeutic activity to treat SARS-CoV-19. An exhaustive compendium on medicinal plants by Ross [9] deals with chemical constituents such as essential oils, antioxidants, antimicrobial properties, and curative agents against various diseases. Besides pharmaceuticals, these plants have found extensive use in cosmetics, agriculture, and food industries [10]. It has been suggested that the decomposition and transformation of medicinal herbs into the vapor phase during wood burning (Yagya) releases many volatile phytochemicals thus spreading fragrance and helping in the purification of the environment [11].
It has been reported that medicinal plants, in general, contain a high concentration of polyphenols, flavonoids, tannins, alkaloids, and other bioactive molecules having high metal binding capacity [12,13]. Also, medicinal plants are known to accumulate many mineral (inorganic) elements derived from the local soil depending on its characteristics and geo-environmental factors [12,14]. Many elements such as Fe, Mn, Co, Cu, Zn, etc, present in soil are likely to be assimilated by plants and the same may be supplied to the human body in bioavailable form without causing any deleterious effects [12]. Only adequate amounts of these micronutrient elements are essential to maintain metabolic functions in our body because its excess or deficiency may cause serious harmful effects resulting in diseases. In the last few decades, the importance of micronutrients and trace elements has been greatly realized in human diseases and their curative effects, Therefore, many workers from different countries have extensively analyzed medicinal herbs and herbal formulations using different techniques such as atomic absorption spectrometry (AAS) [15], energy dispersive X-ray fluorescence (EDXRF) [16], inductively coupled plasma mass spectrometry (ICP-MS) [17], particle-induced X-ray emission (PIXE) [18,19] and instrumental neutron activation analysis (INAA) [12,20-22]. During the last few decades, we have extensively used INAA for the quantification of elemental contents in a variety of medicinal herbs and herbal formulations [23-26].
It is believed that the materials such as sandalwood, mango wood, and giloy stem used in performing Yagya contain many volatile organic compounds (VOCs) which enter the atmosphere when they are burnt in holy fire. Further, these VOCs are likely of medicinal importance when inhaled by the persons performing Yagya. Thus they are likely to become immune to or get cured of various diseases. This has been referred to as smoke therapy as it was practiced in ancient India when the smoke of many herbs was found to be effective as having antibacterial with disinfectant properties [27]. In an earlier study, we have identified three new phytoconstituents in methanolic and hexane extracts of M indicia (mango wood) [28]. GC-MS studies have been used to identify phenol, myristic acid, diethyl phthalate, ethyl vanillin and cyclotene in the fumes extract of T cordifolia [29]. Similarly, for S album, compounds identified are dibutyl phthalate, 2, 4-ditert butyl phenol, cyclotene, phenol, guaiacol, diethylene glycol, and hydroquinone [29]. T cordifolia extract is widely marketed in tablet/powder form in India and is also used in many herbal formulations.
In the present study, we have used INAA for the determination of 4 minor and 13 trace and ultra-trace elements in three stem samples of two trees and a climber using short and long reactor irradiation with neutrons followed by assaying of gamma activity at different intervals by high-resolution gamma-ray spectrometry. Reference Materials (RMs) from the National Institute of Standards and Technology (NIST), USA, and the Institute of Nuclear Chemistry and Technology (INCT), Poland were used as comparator standards. An attempt has been made to interpret elemental contents in terms of curative effects of medicinal plants.
Sample collection and preparation
The dried stem samples of S album, M indica and T cordifolia were procured from Shanti Kunj Pharmacy, Haridwar. These were carefully washed with doubly distilled water to remove any dirt or other surface contaminants. Finally, these were dried at 80 oC in an oven for 24 h, crushed to fine homogeneous powder, and passed through a 100 mesh sieve. Reference Materials NIST SRM 1547 (Peach leaves) [30] and INCT MPH-2 (Mixed Polish Herbs) [31], as comparator standards, were used as received from the respective agencies. All the samples and RMs were dried in an oven at 80 oC for 2 h before packing for irradiation.
Irradiation and counting
About 20 mg each of the samples and RMs were packed in high-density polypropylene rabbits and irradiated at 5 x 1013 ncm-2s-1 for 1 min in a pneumatic carrier facility (PCF) of Dhruva reactor at the Bhabha Atomic Research Centre (BARC), Mumbai. Approximately 100 mg each of samples and RMs were packed in aluminum foil and irradiated at ~1012 n cm-2s-1 for 6 h in the CIRUS reactor of BARC. The gamma activity of the activation products of the samples and RMs was assayed after suitable cooling time using a Compton suppressed gamma-ray spectrometer with 40% efficiency HPGe detector (EG & G ORTEC) having a resolution of 2 keV at 1332 keV of 60Co and coupled to a PC based 8k MCA [12]. All the spectral data were recorded and analyzed using PHAST software in the Radiochemistry Division of BARC. The peak areas under characteristic photo peaks of gamma rays of various radionuclides were used for calculating elemental concentrations using the comparator method of INAA [12].
First, elemental concentrations for the two RMs were calculated using each other as comparator standards at various intervals of cooling. Mean elemental concentrations so obtained for the two RMs, NIST SRM 1547 (Peach Leaves) and INCT MPH-2 (Mixed Polish Herbs) along with their certified/information values [30,31] are listed in Table 1. Standard deviations were calculated based on triplicate analyses. Also listed in Table 1 are Z scores for both the RMs. It is observed that a comparison of our data with the certified values is in good agreement within ± 5% - 10% in all the cases. Further, standard deviations, in all cases, are small and well within ± 10%. Z score values for most elements are within ± 3 except for a few cases. Afterward, elemental concentrations in three samples were calculated using each of the two RMs as comparator standard. Mean elemental concentrations along with standard deviations calculated on the basis of replicate analyses, counting at different intervals, different photo peaks, and using two RMs are listed in Table 2. On the basis of data in Table 1, we can presume that elemental concentrations in samples should also be reliable within ± 5% - 10% limits. A perusal of elemental data in Table 2 shows that some elements are at mg/g level whereas others are at µg/g or ng/g level which may be called minor, trace, and ultra-trace elements respectively. These are discussed separately in the following paragraphs.
| Table 1: Comparison of our data on elemental concentrations in Standard Reference Materials for data validation [30,31]. | ||||||
| Elements | NIST SRM -1547 Peach Leaves | INCT-MPH-2 Mixed Polish Herbs | ||||
| Measured Certified | Z-score | Measured Certified | Z-score | |||
| As (ng/g) | 53 ± 8 | 60 ± 18 | -3.33 | 209 ± 9 | 191 ± 23 | 0.78 |
| Ca (mg/g) | 14.8 ± 1.1 | 15.6 ± 0.2 | -4 | 10.3 ± 0.8 | 10.8 ± 0.7 | -0.71 |
| Ce (µg/g) | 7.99 ± 0.79 | [10] | - | 1.31 ± 0.2 | 1.12 ± 0.10 | - |
| Cl (mg/g) | 0.30 ± 0.01 | 0.36 ± 0.02 | -2.8 | 2.36 ± 0.17 | 2.84 ± 0.20 | -2.4 |
| Co (µg/g) | 0.07 ± 0.06 | [0.07] | - | 0.18 ± 0.02 | 0.210 ± 0.025 | -1.16 |
| Cr (µg/g) | 1.06 ± 0.03 | [1.0] | - | 1.74 ± 0.12 | 1.69 ± 0.13 | 0.38 |
| Cu (µg/g) | 3.66 ± 0.32 | 3.71 ± 0.4 | -0.125 | 6.72 ± 0.48 | 7.77 ± 0.53 | -1.98 |
| Fe (µg/g) | 229 ± 11 | 218 ± 14 | 0.78 | 489 ± 26 | [460] | - |
| Hg (ng/g) | 34.7±2.8 | 31±7 | 0.53 | 20.3 ± 1.7 | 17.6 ± 1.6 | 1.69 |
| K (mg/g) | 24.7 ± 0.2 | 24.3 ± 0.1 | 0.40 | 17.4 ± 1.1 | 19.1 ± 1.2 | -1.42 |
| La (µg/g) | 7.33 ± 0.51 | [9] | - | 0.60 ± 0.03 | 0.571 ± 0.046 | 0.58 |
| Mg (mg/g) | 4.72 ± 0.19 | 4.32 ±0.08 | 3.1 | 2.36 ± 0.21 | 2.92 ± 0.18 | -3.05 |
| Mn (µg/g) | 87.3 ± 7.2 | 98 ± 3 | -3.6 | 183 ± 12 | 191 ± 12 | -0.67 |
| Na (µg/g) | 23.1 ± 1.4 | 24 ± 2 | -0.45 | 381±19 | [350] | - |
| Se (ng/g) | 117 ± 7 | 120 ± 9 | -0.33 | 141 ± 9 | NA | - |
| V (µg/g) | 0.34 ± 0.02 | 0.37 ± 0.03 | -1 | 1.03 ± 0.08 | 0.95 ± 0.16 | 0.5 |
| Zn (µg/g) | 18.7 ± 0.3 | 17.9 ± 0.4 | 2 | 37.7 ± 2.3 | 33.5 ± 2.1 | 2 |
| Values in parenthesis [ ] are for information only. | ||||||
| Table 2: Elemental concentrations in stems of three medicinal plants. | |||
| Elements | Tinospora cordifolia | Santalum album | Mangifera indica |
| As (ng/g) | 400 ± 30 | 630 ± 50 | 230 ± 20 |
| Ca (mg/g) | 12.6 ± 1.1 | 22.7 ± 1.6 | 17.2 ± 1.2 |
| Ce (µg/g) | 1.97 ± 0.06 | 2.45 ± 0.09 | 1.10 ± 0.05 |
| Cl (mg/g) | 4.89 ± 0.14 | 1.77 ± 0.04 | 2.07 ± 0.12 |
| Co (ng/g) | 54.0 ± 3.0 | 56.0 ± 3.0 | 57.0 ± 4.0 |
| Cr (µg/g) | 1.14 ± 0.12 | 1.21 ± 0.09 | 0.97 ± 0.07 |
| Cu (µg/g) | 6.33 ± 0.51 | 4.01 ± 0.42 | 2.39 ± 0.39 |
| Fe (µg/g) | 229 ± 17 | 129 ± 9 | 237 ± 21 |
| Hg (ng/g) | 66.1 ± 1.9 | 71.2 ± 2.3 | 59.7 ± 1.3 |
| K (mg/g) | 24.6 ± 0.3 | 29.3 ± 0.5 | 26.8 ± 0.5 |
| La (µg/g) | 8.42 ± 0.59 | 8.49 ± 0.64 | 4.66 ± 0.31 |
| Mg (mg/g) | 2.15 ± 0.22 | 1.48 ± 0.16 | 0.52 ± 0.08 |
| Mn (µg/g) | 56.7 ± 1.5 | 46.9 ± 1.2 | 38.9 ± 1.1 |
| Na (mg/g) | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.31 ± 0.02 |
| Se (ng/g) | 180 ± 17 | 290 ± 30 | 90 ± 9 |
| V (µg/g) | 0.92 ± 0.05 | 0.61 ± 0.04 | 1.72 ± 0.11 |
| Zn (mg/g) | 34.6 ± 2.1 | 44.6 ± 2.4 | 33.9 ± 2.1 |
| *These mean ± SD values are on the basis of three independent analyses of samples. | |||
Minor elements
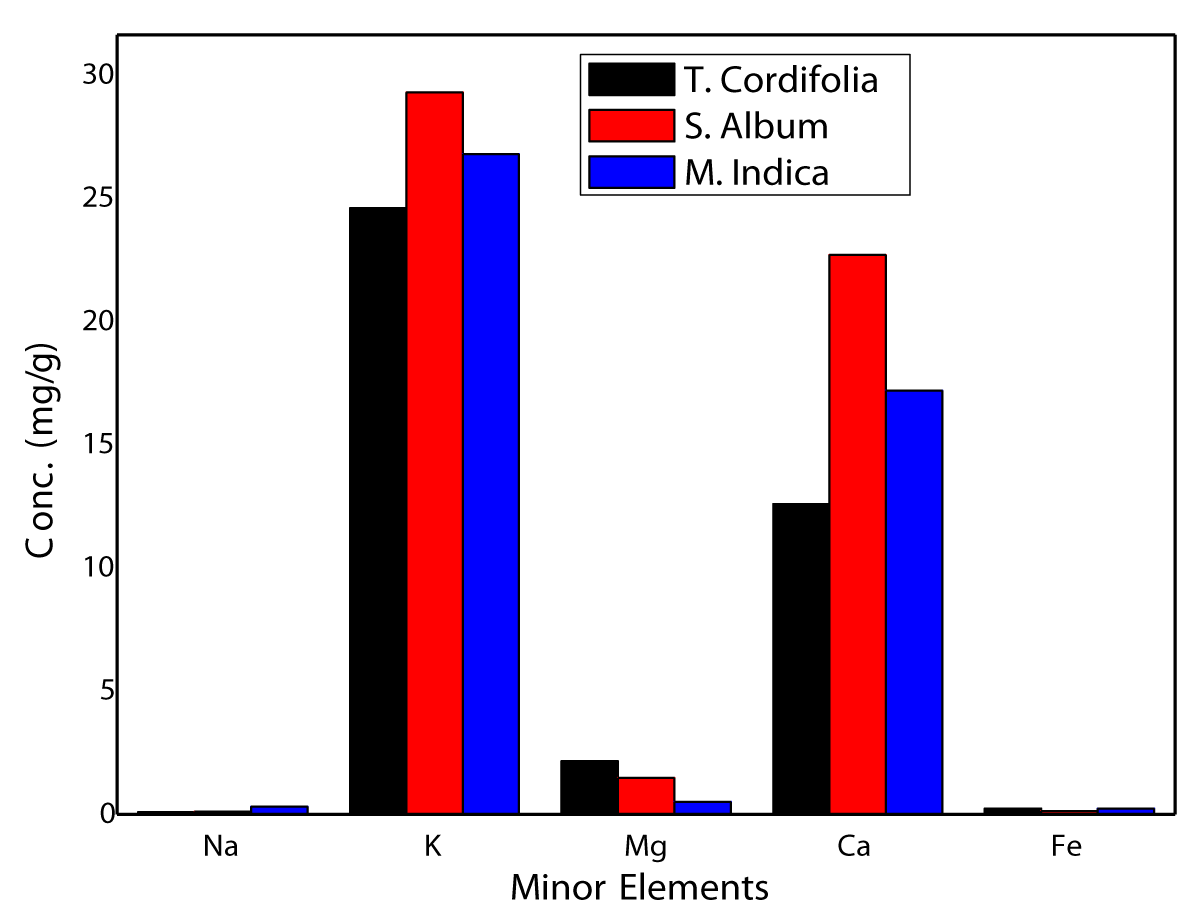
A perusal of data in Table 2 shows that concentrations of K, Ca, Mg and Cl, all electrolytic elements are found at mg/g level and decrease in the order K > Ca > Cl > Mg. The case of Fe is on the borderline as it is found in the range 0.13 - 0.24 mg/g being highest for M indica and lowest for S album. Surprisingly, the concentration of K is highest and varies in a close range of 24.6 – 29.3 mg/g with a mean concentration of 26.9 ± 2.3 mg/g. It suggests that even though all three stems are of different plant species but their K concentration is almost of the same level. However, this is not the case with other minor constituents of Ca, Cl, and Mg which all occur in a much wider range with mean concentrations of 17.5 ± 4.9, 2.91 ± 1.14, and 1.38 ± 0.86 mg/g respectively. A comparison of elemental contents in bar diagram of minor constituents (Na, K, Mg, Ca) including that of Fe is shown in Figure 2, wherefrom it is observed that concentrations of K and Ca are particularly high in all the samples but a decrease in the order S album M indica T cordifolia being highest in sandalwood. On the contrary Na content, at mg/g level, varies in a large range of 0.09-0.31 mg/g with a mean value of ~12 mg/g. However, Mg content is highest in T cordifolia (2.15 ± 0.22 mg/g) and lowest in M indica (0.52 ± 0.08 mg/g). Similarly, concentrations of Cl, an anionic electrolytic element that remains associated primarily with Na and K, show a wider range with the highest content in T cordifolia (4.89 ± 0.14 mg/g) and lowest in S album (1.77 ± 0.04 mg/g). Thus, all three stem samples are enriched in electrolytic elements, irrespective of being cationic or anionic. These play an important role in the transport of liquids along with nutrient elements from root to stem to leaves and flowers.
Figure 2: Bar diagram showing comparative contents for minor constituent elements in M indica, S album and T cordifolia.
A comparison of Ca contents in bark/stem of three samples of this study with that of A indica [26] and T arjuna [32] from our earlier work along with those of other trees from Brazil [33], India [34,35] and Kenya [22] are listed in Table 3. In all of our studies as well as of others on the analysis of bark/stem of trees [22,31-35], Ca content is found to be high in the range 12.6 - 34.1 mg/g or > 1% by weight. Hence K and Ca are in fact major constituents. Lowest Ca content in T cordifolia stem may be attributed to the fact that it is not a tree but a creeper and hence not so strong. Earlier in a separate study, stems of T cordifolia were analyzed in our laboratory [23] whence Ca content was found to be 16.1 ± 0.1 mg/g. Mahima, et al. [35] have reported Ca content of 10.22 mg/g by atomic absorption spectrophotometry (AAS). Thus an overall range of Ca content in T cordifolia is found in a range of 10.2 - 16.1 mg/g with a mean value of 13.0 mg/g. These are quite comparable (within ~20%) considering that all three samples were derived from different sources and analyzed by different workers using different techniques. Ca is a unique essential nutrient linked to transpiration, playing a vital role in plant structure and signaling that regulates water flow [36]. In humans, Ca builds healthy bones and teeth as it is located in the skeleton by virtue of its phosphate salts. It also assists in blood clogging because of its presence in extracellular fluid as a carrier of cellular signals [37]. It may impair the absorption of other elements such as Fe, Mg and Zn. In case of Mg, however, variation is in a much wider range of 0.52 - 2.15 mg/g with a mean concentration of 1.38 ± 0.85 mg/g and decreasing in the order T cordifolia > S album > M indica. It has been suggested that proper Mg2+ homeostasis is compulsory and its deficiency or excess amount may manifest many diseases [38]. As all three stem samples analyzed in this study are enriched in Ca content, these may be used as natural supplements for Ca along with other trace nutrients in bioavailable form.
| Table 3: Comparison of Ca content (in mg/g) in stem/bark of some medicinal plant species from India, Kenya, and Brazil. | ||
| Name of Plant species | Concentration of Ca (mg/g) | Reference |
| Santalum album (Chandana) | 22.7 ± 1.6 | This work |
| Mangifera indica (Mango) Tinospora cordifolia (Giloy) |
17.2 ± 1.2 12.6 ± 1.1 16.3 ± 0.1* 10.22 |
This work This work Garg, et al. [23] Mahima, et al. [35] |
| Azadirachta indica (Neem) | 14.4 ± 5.9* | Garg & Verma, [26] |
| Terminalia arjuna (Arjuna) Erythrina velutina (Mulungu) Salvadora persicawall (Kharajal) Glycyrhiza glabra (Yashtimadhu, Mulethi) |
34.1 ± 11.6* 21.15 ± 0.92 35.10 12.97 ± 0.85 |
Garg, et al. [32] Leal, et al. [33] Benson, [22] Ghosh & Singh, [34] |
| *These values are from our own work reported earlier [23,26, and 32]. In parenthesis ( ) are given local names of the plants. | ||
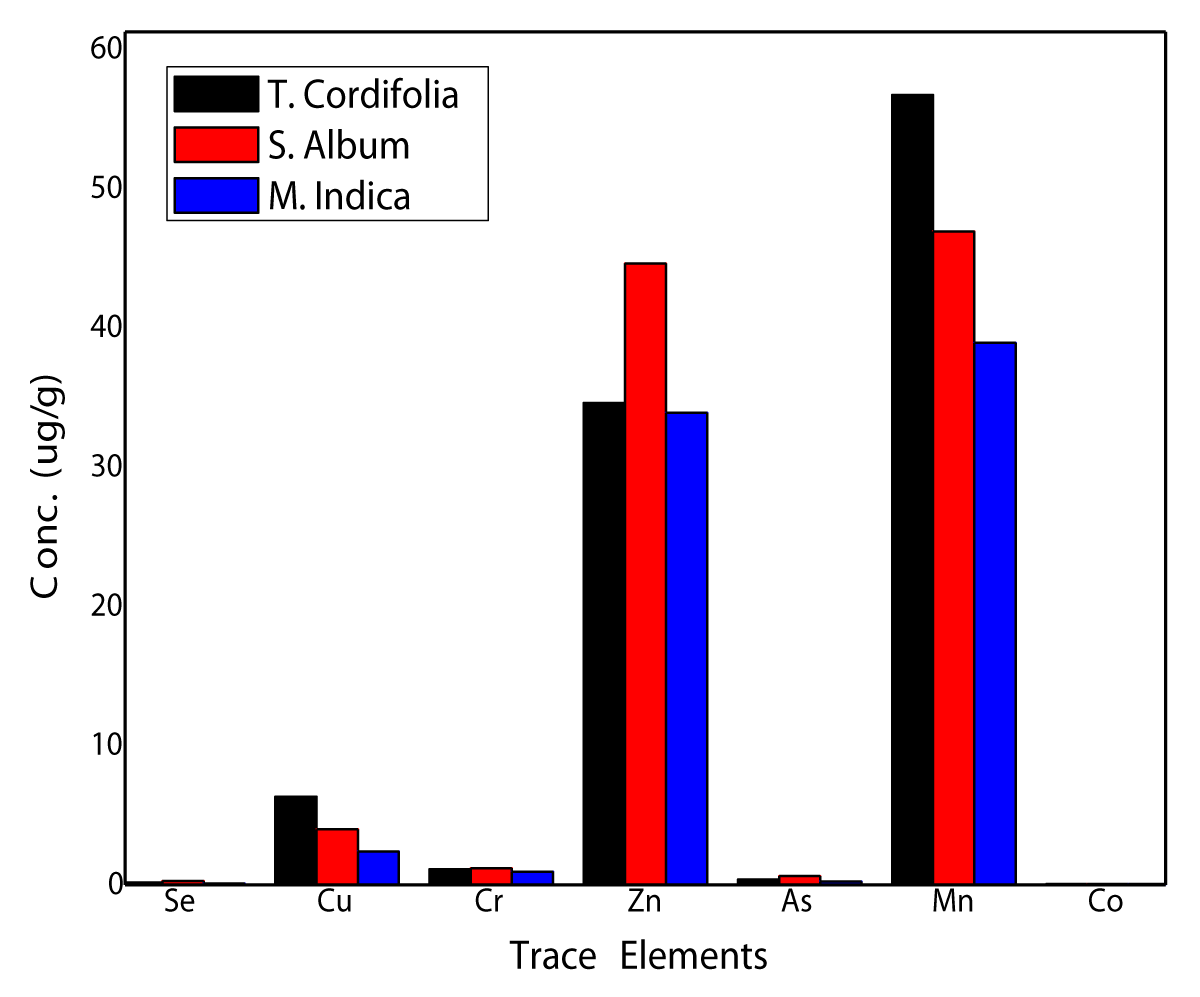
Figure 3: Bar diagram showing variation of trace element contents in stem samples of S album, M indica, and T cordifolia.
Trace elements
Manganese, zinc, copper, chromium, cobalt, vanadium, and selenium are all considered micronutrients and are found in trace amounts at µg/g or ng/g level. It is observed that the concentration of Mn is highest in the range of 40 - 60 µg/g followed by Zn (30 - 45 µg/g), Cu (< 10 µg/g), V (< 2 µg/g), Cr (~1 µg/g), Co (> 50 ng/g) and Se (100 - 300 ng/g). Some of these elements (Se, Cu, Cr, Zn, As, Mn, Co, and Hg) have been plotted as a bar diagram showing a comparison of elemental contents in Figure 3. It is observed that concentrations of Mn, Zn, and Cu, all nutrient elements are particularly high in all three samples but a decrease in the order Mn > Zn > Cu. All three elements are nutritionally important and play a vital role in various biochemical processes in the human body [38-40]. Besides, higher Mn and Zn contents may also be associated with antioxidant properties justifying their use in traditional herbal medicines [4]. Zinc is an essential component of a large number of enzymes such as carboxypeptidase participating in the metabolism of other micronutrients. Out of three plant species, S album is particularly enriched in Zn, Se, and Cr whereas T cordifolia is enriched in Mn and Cu. Gowrishankar, et al. [18] have also analyzed T cordifolia by PIXE and our results are comparable for most elements except Cr and Cu. Mahima, et al. [35] have also reported a few trace elements (Fe, Mn, Cu, and Zn) in T cordifolia by AAS that are somewhat lower. This may probably be attributed to the destructive nature of AAS and the sample being from a different location. Higher contents of Mn and Cu in T cordifolia may be attributed to the extensive medicinal use of this herb in the Ayurvedic health care system. Mn is an essential nutrient because it serves as a cofactor for numerous cellular processes. It activates enzymes such as pyruvate carboxylase and superoxide dismutase essential for the synthesis of polysaccharides and glycoprotein [39] Concentration of Co, however, is at the ultra-trace level (56 ± 2 ng/g) in all three samples. The concentration of V is highest in M indica (1.72 ± 0.11 µg/g) and lowest in S album (0.61 ± 0.04 µg/g). It is considered as a close blueprint of phosphate and is likely to take over a regulatory function in metabolic processes depending on phosphate [37]. Selenium considered an essential element as a part of selenoenzyme glutathione peroxidase is also found at ultra-trace (ng/g) level. It is now considered an essential micronutrient for mammals though it is also proven to be toxic in excess amounts leading to selenosis [38]. It has been proposed that the minor and trace elements remain bound with phytoconstituents such as polyphenols, steroids thus making them bioavailable/bioaccessible to our body system [14,24,40].
Arsenic and mercury, primarily considered toxic elements, are found at 200 - 600 ng/g and 60 - 70 ng/g levels respectively. Arsenic is linked with toxicity and its ability to cause diseases as a potential health hazard. However, there is evidence that it has to sustain a role in living organisms [39]. Mercury is considered highly toxic and poisonous affecting the body's nervous system. It is observed that as and Hg both are within permissible levels [41] and may have been associated with the stem/bark of respective trees as environmental contaminants due to vehicular emissions and spray of insecticides/pesticides. Because of increasing industrial and vehicular emissions, Chen [42] has suggested following good agricultural practices (GAP) and good manufacturing practices (GMP) while preparing herbal medicines and also continuously monitoring these for toxic elemental contents.
Elemental correlations
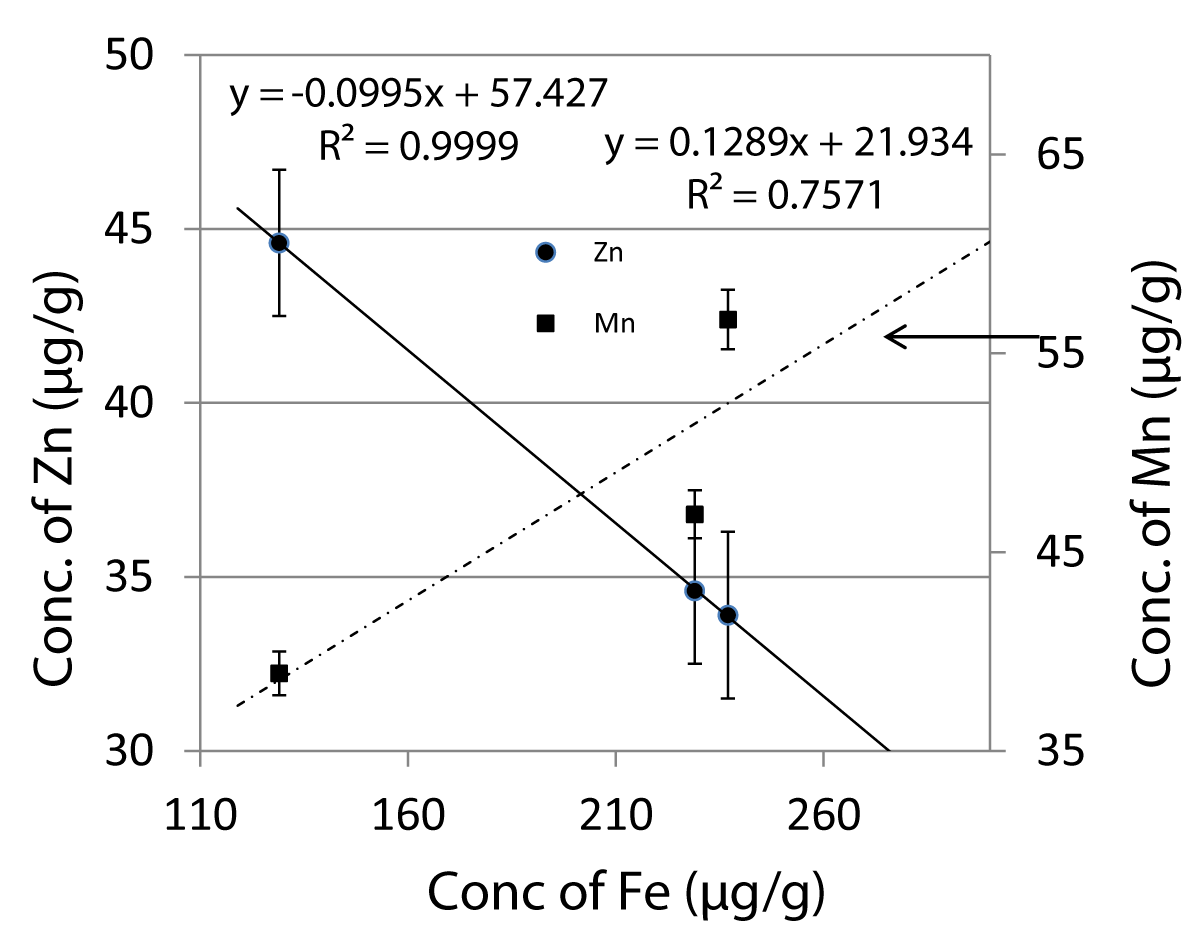
In biological systems, many elemental correlations have been attempted as their interrelations have been correlated with human diseases [37]. Earlier in our laboratory, Choudhury, et al. [43] have studied the interaction of several elements in medicinal herbs used in the treatment of diabetes and observed a statistically significant relationship between Cu and Zn. It has been shown that synergistic interactions occur between essential micronutrient elements showing participation in the same biochemical processes. Recently Konieczynski and Wesolowski [44] have particularly emphasized statistically significant correlation pairs; Zn-Mn, Fe-Zn, Mn-Fe, and Cu-Zn in medicinal plants being used as raw materials. Because of this, we examined several correlations and observed an excellent inverse correlation between Fe and Zn with a high correlation coefficient, R2 = 0.9999 as shown in Figure 4. In the same Figure 4, another correlation between Fe and Mn is attempted but these do not seem to correlate (R2 = 0.7571) at all. Correlation between Fe and Zn indicates that these elements may participate in mutual biochemical reactions in an antagonistic manner. Derkach and Khomenko [45] have also reported a similar correlation between Fe and Zn in three Ukrainian medicinal plants. This may be useful while manufacturing drugs.
Figure 4: Correlation of Fe with Zn and Mn in S album, M indica and T cordifolia showing antagonistic interaction between Fe and Zn.
Three stem samples of S album, M indica and T cordifolia, used as herbal medicines or in the preparations of herbal formulations were analyzed for 4 minor (K, Ca, Cl, Mg) and 13 trace elements (Fe, Zn, Mn, Cu, Cr, Na, La, As, Hg, V, Co, Se, Ce) by INAA. All the minor constituents at mg/g level were found in decreasing order K > Ca > Cl > Mg. Calcium content in all three samples was found comparable with those of stem/bark samples of medicinal plants reported in the literature. Contents of Fe, Mn, Zn, and Cu were in significant amounts with a strong inverse correlation between Fe and Zn (R2 = 0.9999) indicating antagonistic interaction between the two elements. Toxic elements as and Hg were found below the permissible level. Present data on elemental concentrations will help in understanding the role of elements in the medicinal stem samples used in the cure of diseases and the development of new formulations used as natural drugs.
Declarations
Consent for publication: We certify that the research work presented in this paper is our own work and original. Further, it is not submitted anywhere else for publication and has the consent of all the authors.
Availability of data and material: All the data generated or analyzed in this study are contained within this article.
Author’s contribution: A N Garg- Conceptualisation, planning, supervision, writing, reviewing, and editing the manuscript.
Ruchi Singh- Visualisation, sample collection, preparation, data analysis, compilation, and drafting.
R S Maharia- All experimental work at BARC, Mumbai, and data collection.
R K Dutta- Data checking, supervision, and reviewing.
A Datta- Correlation of data, statistical analysis, Figure drawing, and checking.
Award of fellowship to RSM vide (SRF) 09/143(0379)/2007-EMR-1 is thankfully acknowledged. Our sincere thanks to Dr. R Acharya for his help during irradiation work and for providing counting facilities at the Radiochemistry Division, BARC, Mumbai.
- Kapoor LD. Handbook of Ayurvedic medicinal plants, Herbal Reference Library, CRC Press, Boca Raton, Florida. 2000; 424.
- Krotz A, Helmchen G. Total syntheses, optical rotation and fragrance Properties of sandalwood constituents (-)-(z) and (-)-(E)-β-santalol and their enentiomers, ent-β-santalene, Liebigs. Ann Chem. 1995; 601-609.
- Sindhu RK, Upma, Kumar A, Arora S. Santalum album Linn: A review on morphology, phytochemistry, and pharmacological aspects. Intl J Pharm Tech Res. 2010; 2: 914-919.
- Nunez-Selles AJ. Antioxidant therapy: myth or reality? J Braz Chem Soc, 16: 699-710.
- Nadkarni AK. Editor. Dr KM Nadkarni’s Indian Materia Medica. 2011; 2:
- Sharma P, Dwivedee BP, Bisht D, Dash AK, Kumar D. The chemical constituents, and diverse pharmacological properties of Tinospora cordifolia. Heliyon. 2019; 5: e02437. PubMed: https://pubmed.ncbi.nlm.nih.gov/31701036/
- Kapil A, Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacology. 1997; 58: 89-95. PubMed: https://pubmed.ncbi.nlm.nih.gov/9406896/
- Khan MB, Rathi B. Tinospora cordifolia – An immunomodulatory drug in Ayurveda for prevention and treatment of Covid-19. Int J Res Pharm Sci. 2020; 11: 1695-1699.
- Ross IA. Medicinal Plants of the world; chemical constituents, traditional and modern medicinal uses. 2005. 1-3.
- Archana, Jatawa S, Paul R, Tiwari A. Indian medicinal plants: a rich source of natural immunomodulatory. Int J Pharmacol. 2011. 7: 198-205.
- Singh R. Yagya-Vedic way to prevent air pollution Dev Sanskriti Interdisciplinary. Int J. 2012; 1: 28-34.
- Maharia RS, Dutta RK, Acharya R, Reddy AVR. Correlation between heavy metal contents and antioxidant activities in medicinal plants grown in copper mining areas. J Radioanal Nucl Chem. 2012; 294: 395-400.
- Saha A, Pawar VM, Jayraman S. Characterization of polyphenols in Terminalia arjuna. Indian J Pharm Sci. 2012; 74: 339-347. PubMed: https://pubmed.ncbi.nlm.nih.gov/23626389/
- Choudhury RP, Garg AN. Variation in essential, trace and toxic element contents in Murraya Koenigii-A spice and medicinal herb from different Indian states, Food Chem. 2007; 104: A1454-1463.
- Kolasani A, Xu H, Millikan M. Evaluation of mineral content of Chinese medicinal herbs used to improve kidney function and chemometrics. Food Chem. 2011; 127: 1465-1471.
- Ekinci N, Ekinci R, Polat R, Budak G. Analysis of trace elemnts in medicinal plants with energy dispersive X-ray fluorescence. J Radioanal Nucl Chem. 2004; 260: 127-131.
- Laxmi Reddy S, Gangi Reddy NC, Subba Reddy RR, Reddy GS, Reddy BJ, et al Characterization of red sandalwood by ICP-MS analysis, optical absorption and EPR spectroscopic methods. Radiat Effects Def Solids. 2006; 161: 531-537.
- Gowrishankar R, Kumar M, Menon V, Divi SM, Saravanan M, et al. Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa Oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Elem Res. 2010; 133: 357-363. PubMed: https://pubmed.ncbi.nlm.nih.gov/19588079/
- Ibrahim AAM, Etayeb MA, Khalid H, Noun M, Roumie M, et al. PIXE as a complement to ICP-OES trace metal analysis in Sudanese medicinal plants. Appl Radiat Isot. 2014; 90: 218-224. PubMed: https://pubmed.ncbi.nlm.nih.gov/24814608/
- Khalid S, Mouzai M, Arrarem A, Hamidatoo L, Zourannen B. Elemental analysis of traditional medicinal seeds by instrumental neutron activation analysis. J Radioanal Nucl Chem. 2009; 281: 87-90.
- Wasim M, Daud M, Arif M, Islam R, Iqbal S, et al. Characterization of some exotic fruits (morus nigra, Morus alba, Salvadora persica, and Carissa opaca) used as hebal medicines by neutron activation analysis and estimation of their medicinal value. J Radioanal Nucl Chem. 2012; 292: 653-659.
- Benson RA, Mohamed NMA, Soliman M, Hassan M, Abou Mandour MA. Application of k0-INAA for the determination of essential and toxic elements in medicinal plants from West Pokot County, Kenya. J Radioanal Nucl Chem. 2017; 314: 23-29.
- Garg AN, Kumar A, Nair AGC, Reddy AVR. Analysis of some Indian medicinal herbs. J Radioanal Nucl Chem. 2007; 271: 611-619.
- Choudhury RP, Reddy AVR, Garg AN. Availability of essential elements in nutrient supplements used as antidiabetic herbal formulations. Biol Trace Element Res. 2007; 120: 148-162. PubMed: https://pubmed.ncbi.nlm.nih.gov/17916967/
- Garg AN, Kumar A, Nair AGC, Reddy AVR. Elemental analysis of brahmi (Bacopa monnieri) extracts by neutron activation and its bioassay for antioxidant, radioprotective and anti-lipid peroxidation activity. J Radioanal Nucl Chem2009; 281: 53-58.
- Garg AN, Verma K. Radioisotopes in chemical research: neutron activation analysis of leaves and bark of neem. Asian J Chem. 2012; 24: 5435-5440.
- Khedkar S, Goel S, Ojha NK. Ayurveda dhoopana (medicated smoke) chikitsa in present scenario: A review. Int J Res. Ayurveda Pharm. 2015; 7: 98-102.
- Singh R, Singh SK, Maharia RS, Garg AN. Identification of new phytoconstituents and antimicrobial activity in stem bark of Mangiferra indica L. J Pharm Biomed Anal. 2015; 105: 150-155. PubMed: https://pubmed.ncbi.nlm.nih.gov/25555263/
- Singh R. Ph D Dissertation, Study of chemical constituents of Yagya material and its fumes, Dev Sanskriti Vishwavidyalaya, Shantikunj, Haridwar, India. 2017.
- Certificate of Analysis, Peach Leaves, SRM-1547. National Institute of Standards and Technology, USA. 1993;
- Dybczynski R, Danko B, Kulisa K, Chajduk-Masleszewska E, Polkowska-Motrenko H, et al. Final certification of two new Reference Materials for inorganic trace analysis. Chem Anal. 2004; 49: 143-158.
- Garg AN, Gajbhiye PT, Choudhury RP. INAA of essential micronutrients in Terminalia arjuna bark powder: a versatile heart tonic. J Radioanal Nucl Chem 314: 1539-1545.
- Leal AS, Prado G, Bomfim Gomes C, Sepe FP, Dalmazio I. Determination of metals in medicinal plants highly consumed in Brazil. Brazilian J Pharm Sci. 2013; 4: 599-607.
- Ghosh S, Singh M. Minerals and heavy metal contents of Glycyrhiza glabra L. and Andrographis paniculata (Burm F.) from Meerut, India. Int J Pharm Bio Sci. 2015; 6: 40-45.
- Mahima Rahal A, Prakash A, Verma AK, Kumar V, Roy D. Proximate and elemental analyses of Tinospora cordifolia stem. Pak J Biol Sci. 2014; 17: 744-747. PubMed: https://pubmed.ncbi.nlm.nih.gov/26031012/
- Gilliham M, Dyod M, Hocking BJ, Xu B, Conn SJ, et al. Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exptl Botany. 2011; 62: 2233-2250. PubMed: https://pubmed.ncbi.nlm.nih.gov/21511913/
- Shih MC, Chang CM, Kang SM, Tsai ML. Effect of different parts (leaf, stem and stalk) and seasons (summer and winter) on the chemical compositions and antioxidant activity of Moringa oleifera. Int J Mol Sci. 2011; 12: 6077-6088. PubMed: https://pubmed.ncbi.nlm.nih.gov/22016645/
- Siegel A, Siegel H, Siegel RKO. Interrelations between essential metal ions and human diseases, Springer, Heidelberg. 2013; 13.
- O’Dell BL, Sunde RA. Handbook of nutritionally essential mineral elements, Marcel Dekker Inc, New York. 1997;
- Collins JF. Molecular, genetic and nutritional aspects of major and trace metals, Academic Press (Elsevier), New York. 2016.
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, et al. Heavy metal content of Ayurvedic herbal medicine products. J Am Medical Asso. 2014; 292: 2868-2873. PubMed: https://pubmed.ncbi.nlm.nih.gov/15598918/
- Chen K. Some aspects of toxic contaminants in herbal medicines, Chemosphere. 2003; 52: 1361-1371. PubMed: https://pubmed.ncbi.nlm.nih.gov/12867165/
- Choudhury RP, Acharya R, Nair AGC, Reddy AVR. Availability of essential trace elements in medicinal herbs used for diabetes mellitus and their possible correlation. J Radioanal Nucl Chem. 2008; 276: 85-93.
- Konieczynski P, Wesolowski M. Interrelationships among selected essential elements in medicinal plant raw materials and their water extractable forms. Herba Polonica. 2013; 59: 46-57.
- Derkech T, Khomenko V. Elemental composition of the medicinal plants Hypericum perforatum, Urtica dioica and Matricaria chamomilla grown in Ukrain; A comparative study. Pharmacog J. 2018; 10: 486-491.