Research Article
Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed
Upadhyaya H1*, Roy H2,3, Shome S2, Tewari S4, Bhattacharya MK2 and Panda SK5
1Department of Botany, Cotton University, Guwahati-781001, Assam, India
2Department of Botany and Biotechnology, Karimganj College, Karimganj-788710, Assam, India
3Department of Biotechnology, Assam University, Silchar, India
4Department of Physics, Karimganj College, Karimganj-788710, Assam, India
5Plant Molecular Biotechnology Laboratory, Department of Life Science and Bioinformatics, Assam University, Silchar 788011, Assam, India
*Address for Correspondence: Upadhyaya H, Department of Botany, Cotton University, Guwahati-781001, Assam, India, Email: [email protected]
Dates: Submitted: 10 July 2017; Approved: 28 August 2017; Published: 29 August 2017
How to cite this article: Upadhyaya H, Roy H, Shome S, Tewari S, Bhattacharya MK, et al. Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed. J Plant Sci Phytopathol. 2017; 1: 062-070.
DOI: 10.29328/journal.jpsp.1001008
Copyright License: © 2017 Upadhyaya H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Zinc Nanoparticles (Zn NP); Germination; Oryza sativa
Abstract
Nanoparticles affects growth and development of Plant. Zinc is an important micronutrient that regulates various physiological responses in plant. Application of nanoparticles for modulating plants physiological response is a recent practice. Zinc nanoparticles has been widely used in industry for several decades. However, no significant work had been made on its potential use in agriculture. Understanding physiological effect of Zn NP on rice seed germination could suggest the basis for its prospective application in agriculture to improve plant growth. In the present experiment effect of Zn NP was studied in Kmj-6-1-1 which is a commonly growing rice cultivar of Karimganj district of Assam, India. An exposure to Zn NP (0 mg/L, 5mg/L,10mg/L, 15mg/L, 20mg/L & 50mg/L) caused significant changes in radicle and plumule length , mass ( fresh & dry mass) and seed moisture content in rice. Antioxidant enzymes like guaiacol peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD) and gluthathione reductase (GR) also increased due to ZnNP treatment. This suggest that Zn NP may significantly alters antioxidant metabolism during rice seed germination. In conclusion, Zn NP protected rice plants from ROS damage by improving levels of antioxidant enzyme activities during germination. As a consequence the Zn NP treated seeds, showed better potential for germination. Further, genomic analysis of germinating rice seeds are needed to elucidate the molecular mechanisms by which Zn NP modulates germination process in rice.
Introduction
Nanotechnology is a growing field with wide application in the fields of science. The use of nanoparticles in the growth of plants and for modulating its physiological response is a recent practice [1-5]. Zinc nanoparticles has been widely used in industry for several decades. However, no significant work had been made on its potential use in agricultural. Zinc (Zn) is typically the second most abundant transition metal in organisms after iron and the only metal represented in all six enzyme classes. Higher plants generally absorb Zn as a divalent cation (Zn2+), which acts either as the metal component of enzymes or as a functional structural or a regulatory co-factor of a large number of enzymes [6,7]. Zinc is one of the essential micronutrients required for optimum crop growth. Plants take up zinc in its divalent form. At this time it still remains unclear whether this uptake is facilitated as diffusion through membranes specific for zinc ion or whether it is mediated by specific transporter(s). It has been concluded that both mechanisms operate, and about 90.5% of the total zinc required by plants moves towards the roots by diffusion. This lateral movement of zinc is highly dependent upon the soil moisture, and this may be the reason why, particularly in arid and semi-arid areas, zinc deficiency is more frequently seen. In India, Zn is now considered the fourth most important yield-limiting nutrient after nitrogen (N), phosphorus (P), and potassium (K). In India alone, 50% of the soils that groundnut is grown show Zn deficiency, which is causing considerable yield loss. Zinc is required for chlorophyll production, pollen function, fertilization and germination [8]. Zinc plays an important role in biomass production [9]. Zinc in normal soil ranges from 10-300 mgKg-1. The concentration of Zn present in Indian soil varies from 30 to 72 mgKg-1 depending on the type of soil [10,11]. In general, concentration of Zn such as 500 mgKg‑1 may be toxic to crops resulting reduced crop yield [12]. Recent report reveals that in India and Indonesia high zinc rice lines are in advanced stages of evaluation [13,14]. These high zinc line have 18-22 mgKg-1 with acceptable yield potential, grain quality and agronomic traits. Among the micronutrients, Zn and manganese (Mn) can affect the susceptibility of plants to drought stress [15]. However, phytotoxicity of ZnO has been reported [16]. Zinc plays a fundamental role in protecting and maintaining structural stability of cell membranes [17]. Zn is used for protein synthesis, membrane function, cell elongation and tolerance to environmental stresses [17]. Plants emerging from seeds with low Zn have poor seedling vigor and field establishment on Zn-deficient soils. Germination also involves the movement of metal ions like Zn, so that it may be utilized efficiently. Zn is mobilized during germination in rice [18]. In rice, Zn localizes to embryo, endosperm and to the aleurone layer of the seeds. The Zn content is particularly high in embryo [18]. During germination, Zn in the endosperm decreases while in the embryo, high levels of Zn accumulates in the radicle and leaf primordium [19]. With time, Zn increases in the scutellum and the vascular bundle of the scutellum. In the scutellum, Zn accumulates to the endosperm similarly to Fe [18,20]. Zn is distributed in the leaf primordium. Hence it may be concluded that high Zn content in seed could act as a starter fertilizer. Ajouri et al. [21], reported that seed priming with Zn was very effective in improving seed germination and seedling development in barley. These results may indicate that high Zn concentration in seeds has very important physiological roles during seed germination and early seedling growth. Slaton et al. [22], reported that treating rice seeds with Zn greatly increased grain yield and concluded that this type of Zn application method is a very economical alternative to more expensive broadcast Zn fertilizer applications.
There are several reports available on Zn seed coating, which show promise for crop improvement [23,24]. For example, Zn seed pelleting significantly increased seed yield and yield related traits in cowpea (Vigna unguiculata L.). Seed pelleted with ZnSO4 (250 mg kg-1 seed) produced significantly higher 100 seed weights, seed weight/plant leading to 32.1% seed yield increase over non pelleted control [23]. In a series of experiments, seed coating with commercially available formulations ‘Teprosyn-ZnP’ or ‘TeprosynZn’ effectively corrected Zn deficiencies and improved growth and grain yield of sunflower, maize, wheat, soybean and peanut [25]. Thus, Zn plays important role in modulating growth and germination in various plant including rice but there is little or no information on physiological impact of nanosized zinc(Zn NP) on seed germination which is tried to address in the present study. The current study aims to investigate the effect of Zn NP on growth and physiological responses germinating rice seeds. The observation from the present study will prove useful in understanding a possible role of Zn NP in rice during seed germination and will give an insight into role of Zn NP in physiology of rice seed germination.
Materials and Methods
Plant material and treatment
Rice (Oryza sativa L.) seeds were procured from Regional Agricultural Research Station, Akbarpur, Karimganj, Assam. Kmj-6-1-1 seeds were used during experiment. Zn nanoparticle powder (about 30-60nm size) was purchased from Nanoshell cooperation and required amount of Zinc nanoparticle powder was taken to prepare 500mg/ml stock solution, which is used to prepare 0mg/L, 5mg/L, 10mg/L, 15mg/L, 20mg/L, 50mg/L of Zn NP solution.
Seed growth parameters studied
Seed viability test was carried out by the floatation method. The seeds obtained from Regional Agricultural Research Station, Akbarpur, Karimganj, were put in a beaker of water and allowed to stand for five to ten minutes. Seeds that sank were considered viable. About 20 seeds of rice (Kmj-6-1-1) were surface sterilize with 0.1% HgCl2 and wash thoroughly with d.H2O several times. Then the seeds were soaked in different Zn NP suspension (Control, 5mg/L, 10mg/L, 15mg/L, 20mg/L, 50mg/L) for 1h in incubator shaker (160 rpm) in 50ml of solution in conical flask. After 1h plate the seeds in petridish containing moisten filter paper & incubate at 280 C for 48h in dark. After 48h keep the plates under light for another 48h after which germination percentage, seed dry & fresh mass, SMC, PL, RL, PM, RM were measured. All experiments were repeated thrice to five times and data presented mean±SE. The appearance of the plumule at the filter paper surface was taken as germination. Germination % were recorded after 72h of incubation by counting the number of germinating seeds out of total seed plated (25 numbers). After 48h of treatment germinating rice seeds at least 10 seeds per treatment were sampled and radicle and plumule length were measured using centimeter ruler and were separated into radical and plumule and then oven dried at 800C for 48h to estimate the dry mass and expressed in g seed-1. Radicle and plumule ratio was measured by dividing radical length by plumule length. Total dry mass was estimated by adding radicle and plumule dry mass. Total dry mass of seed was measured taking whole germinating seeds and used to calculate RWC of radical and plumule using as per the formula given by Kramer [26].
Antioxidant enzymes analysis
The tissues were homogenized with Tris HCl pH (6.8) 50mM in prechilled mortar and pestle. The extract is centrifuged at 4℃ for 10 min at 10,000 rpm in a cooling centrifuge The supernatant was used for assay of guaiacol peroxidase (GPX), catalase (CAT) and super-oxide dismutase (SOD) and gluthathione reductase (GR) activity assay, The activity of CAT was measured by the method of Aebi [27] and was determined by monitoring the disappearance of H2O2 at 240 nm by using the extinction coefficient 0.036 mM1 cm1. The CAT activity was expressed as µmole H2O2 destroyed min-1g dry weight (DW). GPx activities was assayed as per Chance and Maehly [28]. The activity of SOD was measured using the method of Giannopolitis and Reis [29]. 3.0 ml assay mixture for SOD contained 79.2 mM Tris HCI buffer (pH 8.9), having 0.12 mM EDTA and 10.8 mM tetra ethylene diamine, bovine serum albumin (3.3x10-3%), 6.0mM NBT, 600 µM riboflavin in 5.0mM KOH and 0.2ml tissue extract. Reaction mixture was illuminated by placing the test tubes in between the two tube lights (Philips 20W). Reaction was initiated by switching the light on and terminated after 10 min. The increase in Absorbance due to formazan formation was read at 560 nm. The increase in Absorbance in the absence of enzyme was taken as 100 % and 50 % initial was taken to be an equivalent to 1 unit of SOD activity. Glutathione reductase (GR) was assayed by the method of Smith et al. [30]. The reaction mixture contained 1.0 ml of 0.2M potassium phosphate buffer (pH 7.5) having 1mM EDTA, 0.5ml of 3.0mM DTNB in 0.01M potassium phosphate buffer (pH 7.5), 0.1ml of 2.0mM NADPH, 0.1ml tissue extract and distilled water to make up a final volume of 2.9ml. Reaction was initiated by adding 0.1ml of 2mM GSSG (oxidised glutathione). The increase in Absorbance at 412nm was recorded at 250C over a period of 5min, spectrophotometrically. The activity has been expressed as Absorbance change (∆A412) g. dry mass-1 sec-1.
Data analysis
Each experiment was repeated three to five times with each treatment sample containing ten seeds and data presented are with mean±standard error (SE). The data analysis was carried out using MS excel 2007. LSD test was done to compare significant mean difference from control.
Results and Discussion
In the present investigation Kmj-6-1-1 genotype of rice (Oryza sativa L.) procured from Regional Agricultural Research Station, Akbarpur, Karimganj, Assam, India and collected seeds were used to study the effect of Zn nanoparticles on germination of rice seeds.
Zn NP induced changes in growth of germinating rice seeds
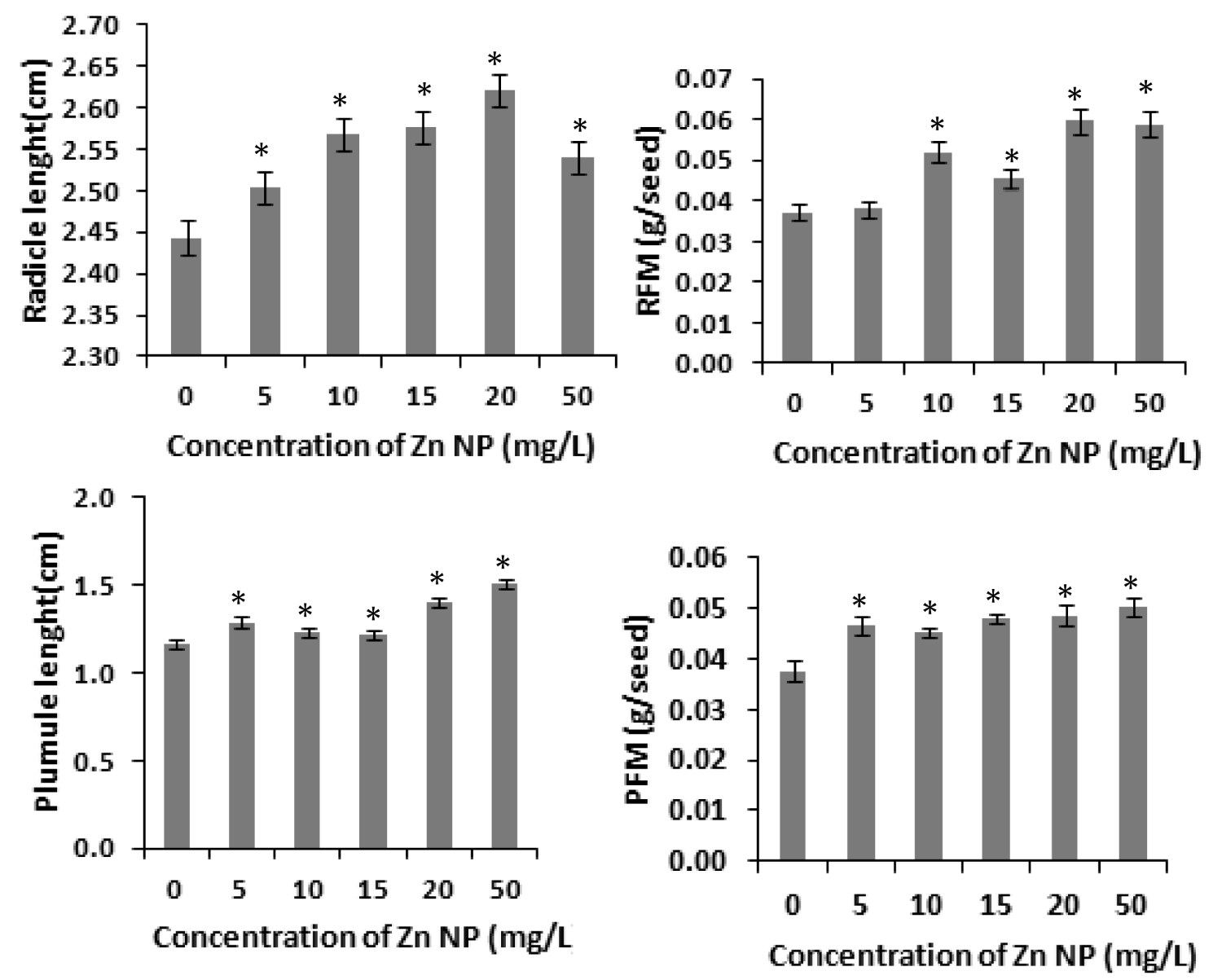
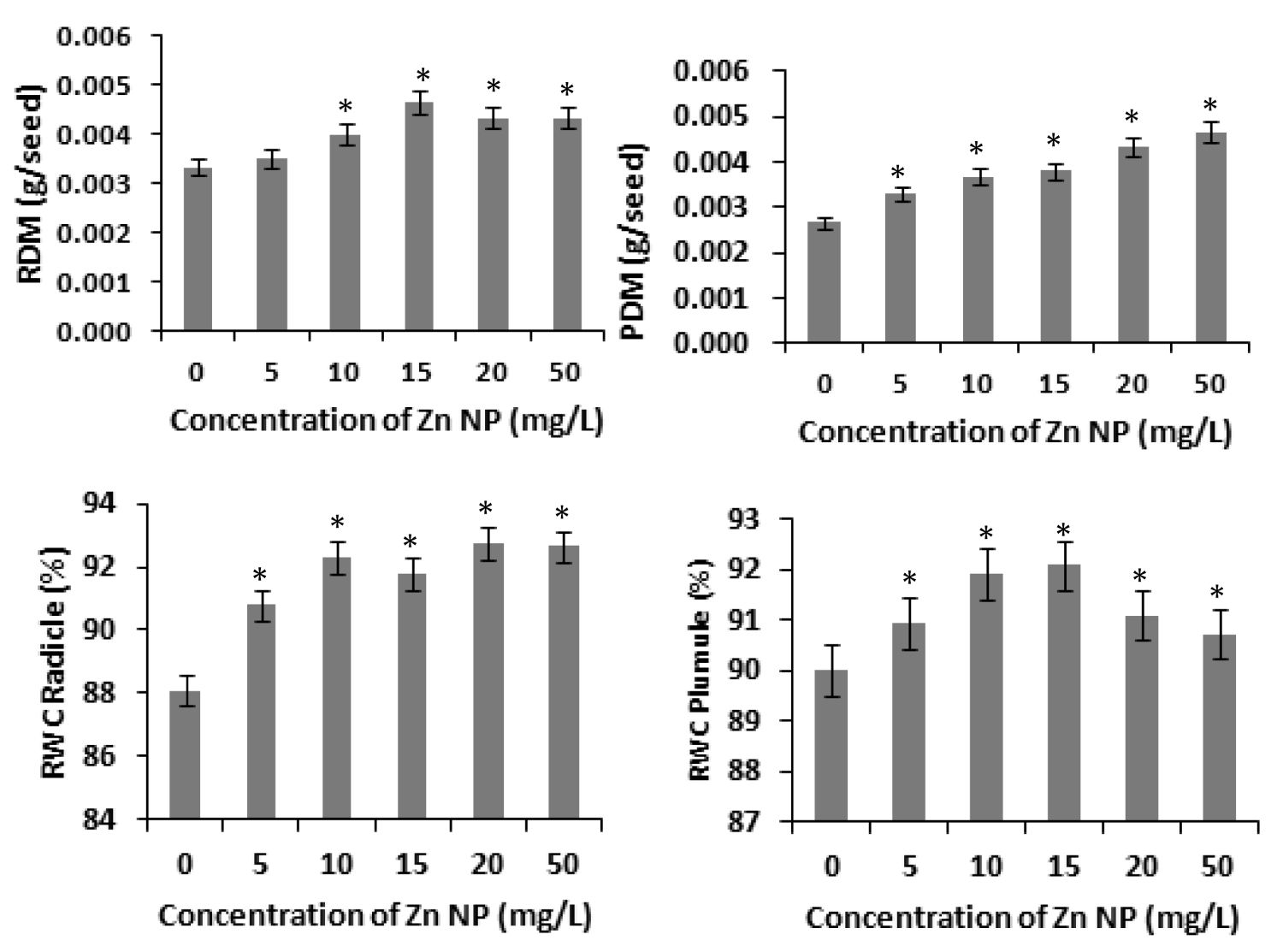
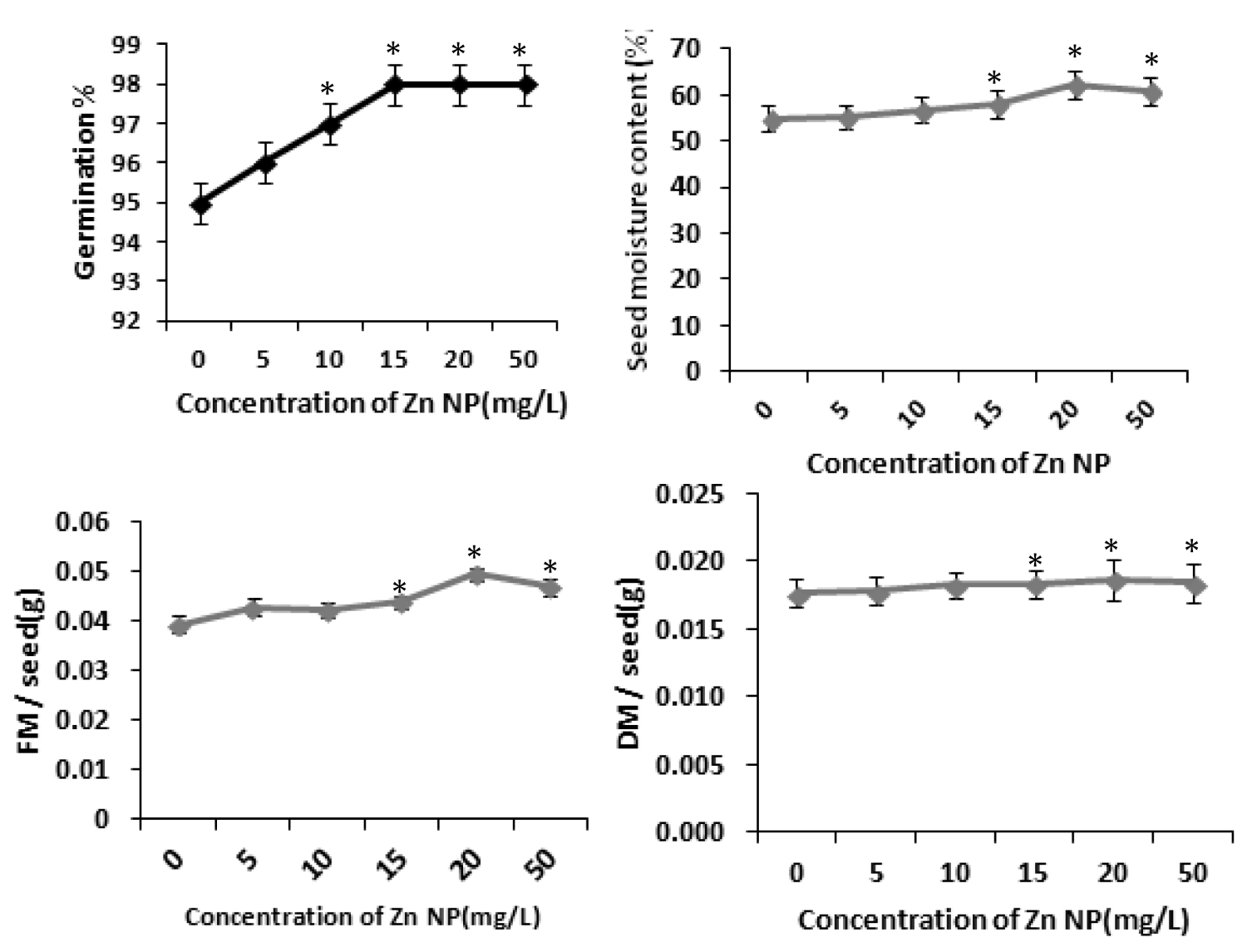
Several studies have shown that zinc affects plant metabolism and its nutrient uptake by roots and other physiological processes [31]. Our results with rice seeds reveals that Zinc nanoparticle (Zn NP) caused increase of radicle and plumule length in rice. As depicted in figure 1, the morphological changes i.e., length of radical and plumule was shown in response to Zn NP treatment when subjected to 0 mg/L,5mg/L,10mg/L,20mg/L and 50mg/L of Zn NP solution. Data obtained by growth analysis of the germinating seeds was very significant. There is gradual increase in the length of the radical and plumule with increase in the concentration of Zn NP solution. As shown in figure 1, Zn NP caused gradual increase in radical and plumule fresh mass as well as dry mass of the germinating rice seed figure 2. As indicated in figure 2, relative water content (RWC) of plumule and radical also slightly increased. The dry mass and fresh mass of the germinating seeds gradual increase along with little increase in seed moisture content (Figure 3). Zn NP promoted seed germination as evidenced from increased rate of seed germination in figure 3, highest rate being shown at 15mg/L which may be attributed to increased moisture content of seeds treated with Zn NP. Earliar report suggest that increased RWC and dry mass is a result of improve growth and recovery in plants and such a process is modulated by Zn [32].
Figure 1: Effect of Zn NP on length and fresh mass of radicle and plumule of germinating rice seeds. Data presented are mean ±SE. * indicate significant mean difference from control by LSD test(p<0.05).
Figure 2: Effect of Zn NP on dry mass and relative water content (RWC )of radicle and plumule of germinating rice seeds. Data presented are mean ±SE. * indicate significant mean difference from control by LSD test(p<0.05).
Figure 3: Effect of Zn NP on total fresh and dry mass, germination percentage and moisture content(SMC) of rice seeds. Data presented are mean ±SE. * indicate significant mean difference from control by LSD test(p<0.05).
Zinc is an essential nutrient that plays important roles in numerous physiological processes in plants, serving as a cofactor for many enzymes and as the key structural motifs in transcriptional regulatory proteins [19]. A deficiency of Zn decreases growth, but excess-Zn is toxic to biological systems through metal-based cytotoxic reactions. Therefore, the uptake and transport of Zn must be strictly regulated. Intracellular Zn homeostasis is achieved through the coordinated regulation of specific transporters engaged in Zn influx, efflux, and intracellular compartmentalization. The level of zinc nutrition may affect plant water relations and alter stomatal conductance. Stomatal conductance and transpiration rates also declined under zinc deficiency. Gas exchange data presented by Hu and Sparks [31], and Sharma et al. [32], indicated that zinc deficiency causes a reduction in the instantaneous transpiration efficiency of leaves. However, zinc fertilization and water stress affects plant water relations has been reported [6]. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species and its effect on plant metabolism has also been well reviewed [8,17,33]. However, little information about the effect of zinc nano particle induced biochemical responses in rice seeds is available. The role of Zn in protecting plant cells from damage by reactive oxygen species may be an important response in seed germination and not simply affecting seed water content, dry matter accumulation and changes in antioxidant balance during germination. Zinc is an indispensible micronutrient which regulates abiotic stress responses in plant. Zinc mitigates arsenic toxicity in plant by modulating ROS and antioxidant function in plant [34]. Zinc nutrition also helps in mitigation of iron toxicity in rice [35]. Zinc nutrition also modulates drought induced growth and antioxidant dysfunction in plant and mitigates dehydration induced damages and improves post stress rehydration responses in plant [30,36].
Zn NP induced antioxidant changes of germinating rice seeds
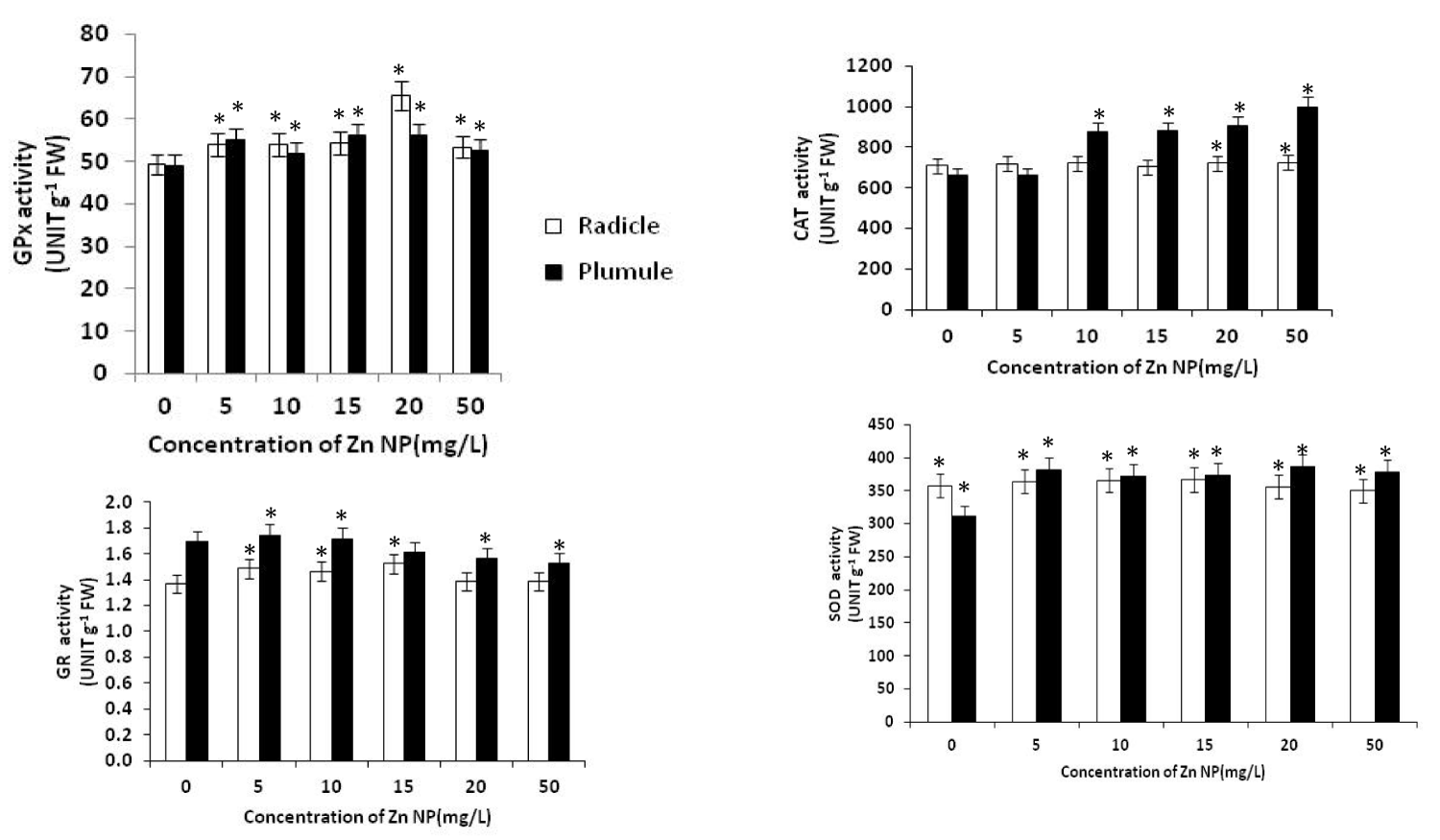
ROS plays a crucial role in causing cellular damage under abiotic stress. Abiotic stress results in increased production of ROS which oxidized target molecules. ROS increases the expression of antioxidant genes leading to increases in the levels of antioxidants and thus enhance ROS scavenging capacity at cellular level confering tolerance against the stress. Secondary products of ROS in plant cells during stress include lipid peroxides and thiol radicals. Although a series of regulatory mechanisms have evolved within the plant cell to limit the production of these toxic molecules, oxidative damage remains a potential problem, since it causes perturbations in metabolism, such as a loss of coordination between energy production (source) and energy utilization (sink) processes during photosynthesis in green leaves in stressful environments. Plants have evolved complex protective mechanisms to prevent the damage initiated by the ROS. The primary constituents include antioxidant enzymes such as SOD, CAT, APX, POX, GR and monodehydroascorbate reductase (MDAR) and free radical scavengers such as carotenoids, ascorbate, tocopherols and oxidized and reduced glutathione (GSSG and GSH) respectively. SOD regulates the cellular concentration of O-2 and H2O2. In the present study, Zn NP treatment showed significant effect on antioxidant enzymes activities. SOD activities were increased in plumule and radicle due to Zn NP treatment (Figure 4). During germination SOD activities increased more significantly in plumule than radical. Increase in SOD activities in germinating seed were the indicative of enhanced O2- production [37], which could be an adaptation to improve growth during germination by regulating ROS level in growing seed. There are many cases that plants growing in hostile environments exhibit increased oxidative stress enzyme activities to combat the deleterious effect of ROS [38,39]. SOD is reported to play an important role in cellular defense against oxidative stress, as its activity directly modulates the amount of O-2 and H2OO-2, the two substrate of the metal catalysed site specific Haber-Weiss reaction resulting in generation of the high reactive OH. SOD catalyses the conversion of the superoxide anion to H2OO-2 and oxygen. CAT activity was increased in both the plumule and radical of growing rice seeds (Figure 4). The ability of growing seeds to enhance CAT activities mediated by Zn NP during germination could improve the process. Increased GR activity in growing seed due to Zn NP treatment, was seen initially in radical while plumule showed decline GR activity during rice seed germination. Glutathione is maintained in a reduced state by GR. However, higher GR activity induced in Zn NP treated plant could be an adaptive advantage for seed growth during germination. GPx activities in the plumule and radicle of germinating seeds increased in response to Zn NP as indicated in Fig. 4. Such increase in GPx activity in both the leaf and root tissues of Vigna radiate [40],
Figure 4: Effect of Zn NP on total fresh and dry mass, germination percentage and moisture content(SMC) of rice seeds. Data presented are mean ±SE. * indicate significant mean difference from control by LSD test(p<0.05).
Conclusion
In conclusion, the effect of Zn NP in the germination of growing seedlings of rice was significant. In the present experiment variation of Zn NP treatment was measured in Kmj-6-1-1 which is a commonly growing rice cultivar of Karimganj district of Assam. An exposure to Zn NP( 5mg/L,10mg/L,15mg/L,20mg/L & 50mg/L) caused significant changes in radicle and plumule length, mass ( fresh & dry mass) and seed moisture content in the tested cultivar of rice. Thus, treating rice seeds with Zn NP protected plants from ROS damage by improving levels of antioxidant enzyme activities. As a consequence the Zn NP treated seeds, showed better potential for germination. Future genomic analysis of germinating rice seeds are needed to elucidate the molecular mechanisms by which Zn NP may mediate germination process in rice seeds.
Acknowledgement
The authors express their sincere thanks to the DBT, Govt of India, for funding DBT RGYI Project (SAN No.102/IFD/SAN/1716/2013-2014 dated July15 2013). We further extend our gratitude to Dr B P Baruah, Chief Scientist, RARS, Akbarpur, and Karimganj for supplying rice seeds throughout the experiment.
References
- Boonyanitipong P, Kositsup B, Kumar P, Baruah S, Dutta J. Toxicity of ZnO and TiO2 Nanoparticles on Germinating Rice Seed Oryza sativa L. Int J Biosci Biochem Bioinfo. 2011; 1: 282.
- Mahajan P, Dhoke SK, Khanna AS. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram (Cicer arietinum) Seedlings Using Plant Agar Method.J Nanotech. 2011; 1. Ref.: https://goo.gl/UDCqD5
- Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, et al. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr. 2012; 35: 905-927. Ref.: https://goo.gl/RvxzfS
- Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, et al. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics. 2014; 6: 132-138. Ref.: https://goo.gl/gZW1L9
- Zhang Z, He X, Zhang H, Ma Y, Zhang P, et al. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics.2011; 3: 816-822. Ref.: https://goo.gl/vMQnfQ
- Khan HR, Mc Donald GK, Rengel Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arientinum L.). Plant Soil. 2004; 267: 271-284. Ref.: https://goo.gl/NsYTwL
- Hafeez B, Khanif YM, Saleem M. Role of Zinc in Plant Nutrition. Am J Expt Agrl.2013; 3: 374-391. Ref.: https://goo.gl/qY8bCT
- Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008; 302: 1-17. Ref.: https://goo.gl/q9KNHp
- Kaya C, Higgs D, Burton A, Phosphorus acid phosphates enzyme activity in leaves in leaves of tomato cultivars in relation to Zn supply. CommunSoil Science Plant Ana. 2000; 31: 3239-3248. Ref.: https://goo.gl/wDBAEq
- Katyal JC, Sharma BD. DTPA extractable and total Zn, Cu, Mn and Fe in Indian soils. Geoderma. 1991; 49: 165-179. Ref.: https://goo.gl/QmVjTA
- Sharma A, Patni B, Shankhdhar D, Shankhdhar SC. Zinc-an indispensable micronutrient. Physiology and Molecular Biology of Plants. 2013; 19: 11-20. Ref.: https://goo.gl/fiuP85
- Alloway BJ. Zinc in Soils and Crop Nutrition. 2nd Edition, Brussels-Paris IZA and IFA. 2008.
- Swamy BPM, Inabangan-Asilo MA, Amparado A, Manito C, Reinke R. Progress in development of high grain Zinc rice varieties for Asia. International Zn Symposium. 2015; 32-33.Ref.: https://goo.gl/tnjLZs
- Swamy BM, Rahman MA, Inabangan-Asilo MA, Amparado A, Manito C, et al. Advances in breeding for high grain Zinc in Rice. Rice. 2016; 9: 49. Ref.: https://goo.gl/kTKHFp
- Khan HR, McDonald GK, Rengel Z. Zinc fertilization improves water use efficiency, grain yield and seed Zn content in chickpea. Plant Soil. 2003; 249: 389-400. Ref.: https://goo.gl/k1WNBm
- Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Envt Poll. 2007; 150: 243-250. Ref.: https://goo.gl/QJZ9cS
- Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000; 146: 185-205. Ref.: https://goo.gl/gw6bvN
- Takahashi M, Nozoye T, Kitajima B, Fukuda N, Hokura N, et al. In vivo analysis of metal distribution and expression of metal transporters in rice seed during germination process by microarray and X-ray Fluorescence Imaging of Fe, Zn, Mn and Cu. Plant Soil. 2009; 325: 39. Ref.: https://goo.gl/8RhrsH
- Ishimaru Y, Bashir K, Nishizawa NK. Zn Uptake and Translocation in Rice Plants. Plant Soil. 2011; 6: 1591-1593. Ref.: https://goo.gl/SFFZQC
- Bashir K, Ishimaru Y, Nishizawa NK. Iron uptake and loading into rice grains. Rice. 2010; 3: 122-130. Ref.: https://goo.gl/88WrTt
- Ajouri A, Haben A, Becker M. Seed priming enhances germination and seedling growth of barley under conditions of P and Zn deficiency. J Plant Nutrition Soil Sci. 2004; 167: 630-636. Ref.: https://goo.gl/Fa64Sr
- SlatonNA, Wilson-Jr CE, Ntamatungiro S, Norman RJ, Boothe DL. Evaluation of zinc seed treatments for rice. Agron J. 2001; 93: 152-157. Ref.: https://goo.gl/juP7aw
- Masuthi DA, Vyakaranahal BS, Deshpande VK. Influence of pelleting with micronutrients and botanical on growth, seed yield and quality of vegetable cowpea, Karnataka. J Agric Sci. 2009; 22: 898-900. Ref.: https://goo.gl/Q9q2UC
- Singh MV. Efficiency of seed treatment for ameliorating zinc deficiency in crops. In: Zinc Crops. Improving Crop Production and Human Health. 2007; 24-26.
- Kramer PJ. Water relation of plants and soils. Academic Press. 1995; 495. Ref.: https://goo.gl/jdduRy
- Aebi H. Catalase in vitro. Methods Enzymol.1984; 105: 121-126. Ref.: https://goo.gl/6iJ7cE
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955; 2: 764.
- Giannopolitis CN, Ries SK, Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997; 59: 309-314. Ref.: https://goo.gl/5WQYA8
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal Biochem. 1988; 175: 408-413. Ref.: https://goo.gl/ULjk6J
- Upadhyaya H, Dutta BK, Panda SK. Zinc modulates drought induced biochemical damages in tea [Camellia sinensis( L) O Kuntze]. J Agric Food Chem. 2013; 61: 6660-6670. Ref.: https://goo.gl/LwkPxK
- Hu H, Sparks D. Zinc deficiency inhibits chlorophyll synthesis and gas exchange in ‘Stuart’ pecan. Hortic Sci. 1991;26: 267-268. Ref.: https://goo.gl/UGLhXj
- Sharma PN, Kumar N, Bisht SS. Effect of zinc deficiency on chlorophyll contents, photosynthesis and water relations of cauliflower plants. Photosynthetic. 1994; 30: 353. Ref.: https://goo.gl/6v1joZ
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytol. 2006; 173: 677-702. Ref.: https://goo.gl/5VYYXR
- Das I, Sanyal SK, Ghosh K, Das DK. Arsenic mitigation in soil-plant system through zinc application in West Bengal soils. Bioremediation Journal. 2016; 20: 24-37. Ref.: https://goo.gl/AH1BkH
- Shahid M, Nayak AK, Shukla AK, Tripathi R, Kumar A, et al. Mitigation of Iron Toxicity and Iron, Zinc, and Manganese Nutrition of Wetland Rice Cultivars (Oryza sativa L.) Grown in Iron‐Toxic Soil. CLEAN-Soil. Air, Water. 2014; 42: 1604-1609. Ref.: https://goo.gl/WaqS4f
- Upadhyaya H, Dutta BK, Panda SK. Impact of zinc on dehydration and rehydration responses in tea. Biologia Plantarum. 2017; 61: 1-4. Ref.: https://goo.gl/hfRnG2
- Asada K, Takahashi M. Production and Scavenging of active oxygen in photosynthesis. Photoinhibition. 1987; 227-287. Ref.: https://goo.gl/KML6aD
- Jebara S, Jebara M, Limam F, Aouani ME. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol. 2005;162: 929-936. Ref.: https://goo.gl/QEdn3J
- Moradi F, Ismail AM. Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-scavenging Systems to Salt Stress during Seedling and Reproductive Stages in Rice. Ann Bot. 2007; 99: 1116-1173. Ref.: https://goo.gl/qbLzMj
- Panda SK, Response of green gram seeds under salinity stress. Indian J. Plant Physiol. 2001; 6: 438-440. Ref.: https://goo.gl/cB4ZWw
- Koji Y, Shiro M, Michio K, Mitsutaka T, Hiroshi M. Antioxidant capacity and damages caused by salinity stress in apical and basal regions of rice leaf. Plant Prod Sci. 2009; 12: 319-326. Ref.: https://goo.gl/LWKUVY