Research Article
Effects of Vochysia haenkeana extract on the neuromuscular blockade induced by Bothrops jararaca venom on chick biventer cervicis preparation in vitro
Fernanda Dias da Silva1, Natália Tribuiani2, Isadora Caruso Fontana Oliveira2, Regina Yuri Hashimoto Miura1, Rafael S Floriano3, Márcio Galdino dos Santos4, Sandro Rostelato-Ferreira1 and Yoko Oshima-Franco2*
1Institute of Health Sciences, Universidade Paulista (UNIP), Sorocaba, SP, Brazil
2Post-Graduate Program in Pharmaceutical Sciences and Veterinary Medicine Graduate Course, University of Sorocaba (UNISO) Sorocaba, SP, Brazil
3Department of Pharmacology, Faculty of Medical Sciences, State University of Campinas (UNICAMP), Campinas, SP, Brazil
4Department of Pharmacology, Faculty of Medical Sciences, State University of Campinas (UNICAMP), Campinas, SP, Brazil
*Address for Correspondence: Dr. Yoko Oshima-Franco, Post-Graduate Program in Pharmaceutical Sciences and Veterinary Medicine Graduate Course, University of Sorocaba (UNISO) Sorocaba, SP, Brazil, Email: [email protected]
Dates: Submitted: 13 July 2017; Approved: 04 August 2017; Published: 08 August 2017
How to cite this article: da Silva FD, Tribuiani N, Oliveira ICF, Miura RYH, Floriano RS, et al . Effects of Vochysia haenkeana extract on the neuromuscular blockade induced by Bothrops jararaca venom on chick biventer cervicis preparation in vitro. J Plant Sci Phytopathol. 2017; 1: 052-058.
DOI: 10.29328/journal.jpsp.1001006
Copyright License: © 2017 da Silva FD, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
SUMMARY
Vochysia haenkeana extract (Vh-E) was assessed against the neuromuscular blockade induced by Bothrops jararaca venom on chick biventer cervicis (BC) preparation. Pre- and post-venom incubation treatments (Pre-vit and Post-vit) were analysed here. Contractures ACh (110 µM) and KCl (20 mM) were evoked before and after addition of venom without stimulation. Vh-E (600 µg/mL) under Pre-vit was more efficient to neutralize the neuromuscular blockade by venom (40 µg/mL) [72.5±4.6% (venom) vs. 45.2±14% (Vh-E) of blockade, p<0.05, n=4]. Vh-E (600 µg/mL) did not cause significant changes under Post-vit [72.5±4.6% (venom) vs. 63.4±8.2% (Vh-E) of blockade, n=4]. The Pre-vit inhibited the blockade of the contracture to ACh (106±17% of response; n=4) while the Post-vit was able to attenuate the effect of the venom on this contracture (55±5% of response; n=4); related to those contractures to KCl both of treatments with Vh-E attenuated the blocker effect of the venom (62.5±7.7% and 55±5% of response for Pre-vit and Post-vit, respectively; n=4). In conclusion, Vh-E neutralizes partially the neuromuscular blockade in Pre-vit, an effect that can be related to preserved function of “extrinsic” post-synaptic receptors, by measured contractures in response to ACh. The myotoxicity of the venom was significantly reduced by Vh-E in both, Pre-vit and Post-vit, by measured contractures in response to KCl.
INTRODUCTION
Accidents by snake bites are considered a severe problem of public health in many regions of the world mainly in sub- and tropical countries. Although the serum therapy is an efficient and recommended treatment, the investigation of alternative methods to delay the effects of the envenomation, especially during the first hours, has proved to be relevant [1]. The effects of medicinal plants against snake venoms have been studied as an alternative to support the serum therapy [2-4]. It has been shown that the bioactive compounds from these plants may neutralize the neuromuscular blockade caused by Bothrops venoms in vitro [5]. In Brazil, especially in some areas of difficult access in the northern of the country, V. haenkeana has been popularly used to treat snake bites but without any scientific evidences [6]. In this work, we have investigated the ability of the hydroalcoholic extract from stem barks of V. haenkeana to neutralize the neuromuscular effects induced by Bothrops jararaca venom on avian neuromuscular preparation in vitro.
MATERIALS AND METHODS
Animals
The animals were housed (5 animals per cage) at 23-25°C on a 12 h light/dark cycle and they had water ad libitum and access to appropriate food (Prefran®, Sales Oliveira, SP, Brazil). This project was approved by the institutional Committee for Ethics in Research of University of Sorocaba (UNISO, protocol no. 53/2015) and the experiments were carried out as the established guidelines of Brazilian Society of Laboratory Animal Science (SBCAL).
Extract of V. haenkeana
The hydroalcoholic extract from stem barks of V. haenkeana (Vh-E) was kindly donated by Prof. Dr. Márcio Galdino dos Santos from Tocantins Federal University (UFT, Porto Nacional, TO, Brazil). The methodology used to obtain Vh-E is essentially described elsewhere [7]. A voucher specimen 10.074 was identified by Solange de Fátima Lolis from the Biological Sciences Department, of the Tocantins Federal University (UFT, Porto Nacional, TO, Brazil) and deposited in the Herbarium of this university in accordance with the International Code of Botanical Nomenclature (ICBN). In all experiments, the Vh-E was solubilized in 30 µL of 70% ethanol (Cinética Soluções Químicas, Londrina, PR), as described elsewhere [8].
Bothrops jararaca venom
B. jararaca venom was donated and certified by Prof. Dr. Stephen Hyslop from the Department of Pharmacology, Faculty of Medical Sciences, State University of Campinas (UNICAMP).
Chick biventer cervicis (BC) preparation
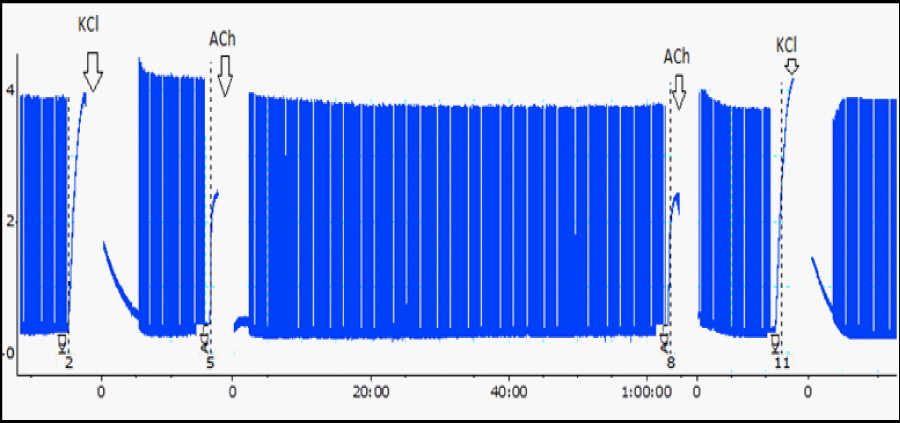
Chicks were killed by halothane inhalation and the biventer cervicis muscles were removed [9] and mounted under a tension of 1 g in a 5 mL organ bath (Panlab® four chamber organ bath) containing aerated (95% O2/5% CO2) Krebs solution (composition, in mM: NaCl 118.1, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaHCO3 25 and glucose 11.1, pH 7.5) at 37°C. A bipolar platinum rings electrode was positioned around the tendinous portion of the muscle and field stimulation was done using a Panlab® LE12406TC stimulator (0.1Hz, 0.2ms, 5-12V) and the myographical records were isometrically obtained via MLT0201 model force-displacement transducers coupled to software-controlled model FE224 DC bridge transducer amplifiers (all from ADInstruments). Data acquisition was done using a PowerLab 4/35 system containing a LabChart and LabChart Pro software (PL3504/P) connected to a LE124060M Power Unit (ADInstruments). The preparations were stabilized for at least 20 min before addition of 110 μM acetylcholine (ACh) or 20 mM potassium chloride (KCl) at the beginning and at the end of each experiment [5], (Figure 1). The muscle response to exogenous agonist is a contracture for as long as the depolarizing agent remains activating the receptors, while the amplitude of the tension response is related to the number of receptors occupied by the drug [10].
Figure 1: A typical myographic register from BC preparation [5], at two moments: under indirect stimulation (see twitches) and with absence of field stimulation (see contractures to ACh and KCl addition, before and after the experiment). Initial twitches mean the control, which amplitude represents 100%. Every 10 min the amplitude was measured and converted to percentage. Arrows, time addition (ACh or KCl). Tension, 1 g.
Experimental protocols
All experiments were carried out during 120 min and consisted of: Krebs control; B. jararaca venom (40 µg/mL, n=6), concentration-response curve of Vh-E (200, 400 and 600 µg/mL, n=5 each) resulting in the Vh-E concentration selection (600 µg/mL), preincubation (Pre-vit, n=4); and post venom (Post-vit, n=4) assays.
STATISTICAL ANALYSIS
The results were expressed as the mean±SEM. The number of experiments (n) is indicated in the legends of their respective figures. The results were analysed statistically using Student’s t-test with the confidence level set at 5% (p<0.05).
RESULTS AND DISCUSSION
The medicinal properties of Vochysia haenkeana (Spreng.) Mart. (Vochysiae) have been related to antimicrobial activities and for treating respiratory diseases [11], with no toxicity to humans reported in the literature. Lima et al. [12], described the leishmanicidal activity of Vh-E against promastigotes of Leishmania amazonenses as being 85.1±0.058 µg/mL. In this work, we have assessed the ability of V. haenkeana extract (Vh-E) to neutralize the effects caused by Bothrops jararaca venom in vitro on chick biventer cervicis (BC) preparation. The venom of B. jararaca is known to induce severe local and systemic effects such as tissue damage, haemorrhage, coagulopathies, hypotension and renal cortical necrosis [13].
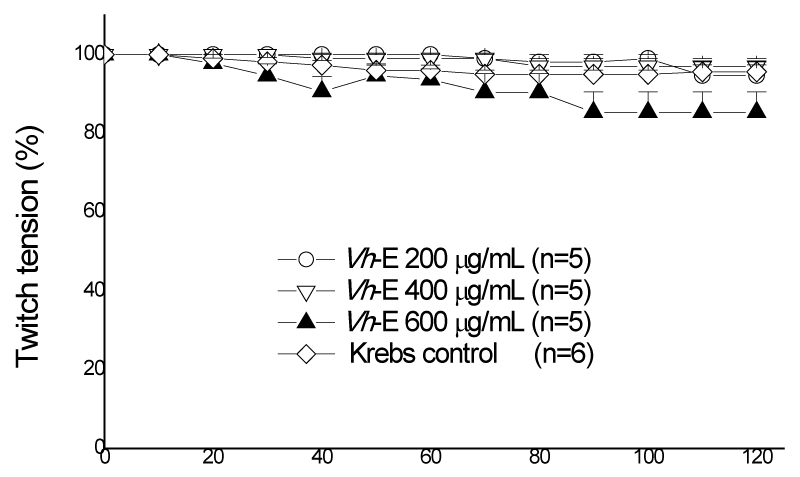
Figure 2 shows a concentration-response curve using 200, 400 and 600 µg/mL (n=5) of Vh-E, all tested concentrations there was no statistical difference compared to those preparations maintained in Krebs solution alone or among themselves. Based on these results, we select the major concentration of Vh-E (600 µg/mL) to be used under pre- and post-venom incubation treatments (Pre-vit and Post-vit). B. jararaca venom (40 µg/mL) induced 72.5±4.6% of blockade compared to control preparations after 120 min incubation (p<0.05, n=6). Zamunér et al. [27], using 50 µg/mL of B. jararaca venom had a time of 50% neuromuscular blockade (T50%) in 107.3±2.6 min, while the concentration of 40 µg/mL venom used in this work had T50% in 80 min, considered ideal for further neutralizing assays.
Figure 2: Chik biventer cervicis preparation, under field stimulation. All tested concentrations of Vh-E were statistically different from Krebs control, but not between themselves. Each point represents the mean±SEM of the number of experiments (n) showed in the legend. Vh-E, Vochysia haenkeana extract.
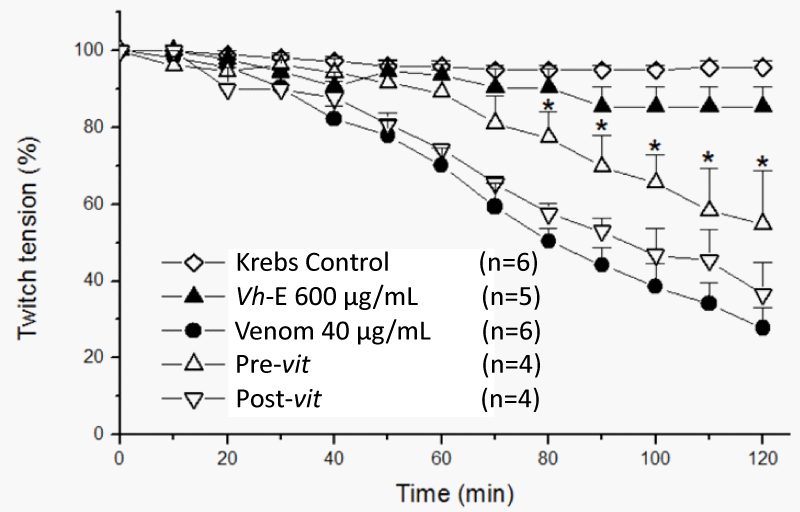
Vh-E under Pre-vit attenuated the neuromuscular blockade by B. jararaca venom (72.5±4.6% vs. 45.2±14% of blockade for venom alone and Pre-vit, respectively, p<0.05, n=4-6), however, the Post-vit was not able to avoid the neuromuscular blockade caused by B. jararaca venom (72.5±4.6% vs. 63.4±8.2% of blockade for venom alone and Post-vit, respectively, n=4-6) (Figure 3). The most studied antiophidian plants at the neuromuscular junction exhibits an in vitro ability in counteracting the neuromuscular blockade-induced by venoms, such as: Casearia sylvestris Sw. [14,15], Plathymenia reticulata Benth. [16], Mikania laevigata Sch. Bip. ex Baker [8,17], Dipteryx alata Vogel [18], Camellia sinensis L. [19,20], Hypericum brasiliense Choisy [21], Vellozia flavicans Mart. Ex Schult. [22], but few plants have been studied by its ability in counteracting the toxic effects after the start of venom action, like as Casearia gossypiosperma Briquet [23,24], Jatropha elliptica (Pohl) Oken. [25] and Terminalia fagifolia Mart. [22]. Post-vit assays allows to show the capacity of certain plants in reaching the local where the snake venom is and avoiding the paralysis evolution [25].
Figure 3: Chick biventer cervicis preparation, under field stimulation. All tested protocols were statistically different from Krebs control. Only Pre-vit protocol was statistically significant in comparison to venom (*p<0.05), with 54.8±14% functioning fibers at the end of experiment, while only 36.6±8.2% (p>0.05 when compared to venom) were active in the Post-vit protocol. Each point represents the mean±SEM of the number of experiments (n) showed in the legend. Venom, Bothrops jararaca venom. Vh-E, Vochysia haenkeana extract.
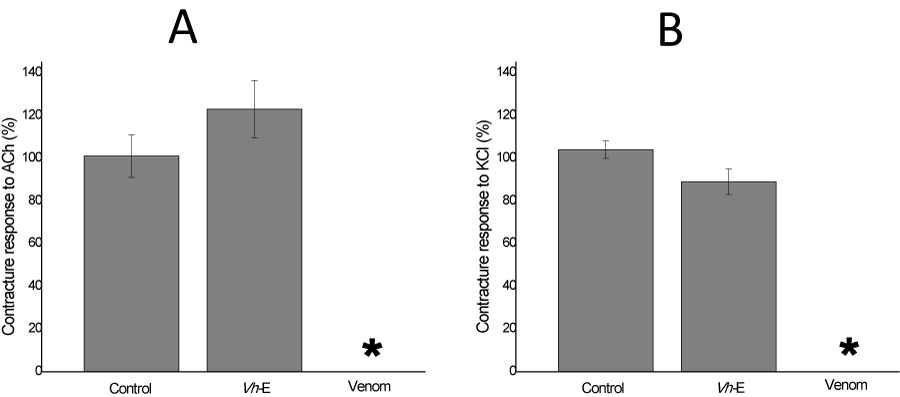
BC preparations with their multiple innervated fibers allow to induce contracture responses to exogenous ACh and KCl without electrical stimulation in order to assess the function of the post-synaptic receptors and muscle membrane integrity, respectively [26]. In those BC preparations incubated with Vh-E alone, there was no statistically change on the muscle contracture responses to exogenous ACh and KCl (Figure 4A), in addition, B. jararaca venom abolished both of contracture responses after 120 min incubation (Figure 4B). Envenomation by B. jararaca venom induce local and systemic myotoxicity and it has already been described its myotoxicity in vitro [10,27].
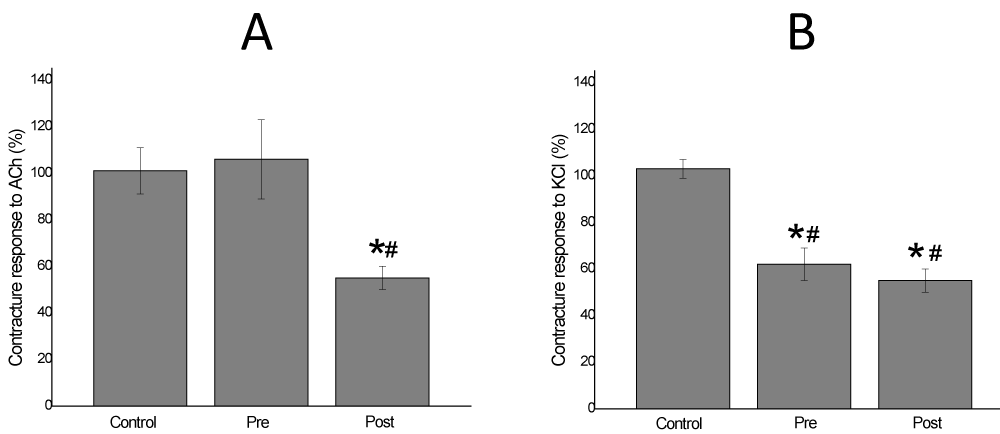
Figure 4: Chick biventer cervicis preparation, in absence of electrical stimulation. Exogenous ACh (A) and KCl (B) added at the end of experiment (120 min) show a responsive contracture of Krebs control of 101±10% and 104±4%, respectively (n=6). V. haenkeana extract (Vh-E) did not alter significantly these parameters, but notice the effect of the venom (B. jararaca) abolishing totally the contracture response of them (n=6). *p<0.05 compared to control
Vh-E through Pre-vit was able to inhibit completely the effect of B. jararaca venom on the contracture response to exogenous ACh (106±17% of response, p<0.05), in addition, Vh-E under Post-vit attenuated the blockade induced by venom on the contracture to ACh (55±5% of response, p<0.05) (Figure 5A). Vh-E under both of treatments attenuated significantly the effect of B. jararaca venom on contracture responses to KCl (62.5±7.7% and 55±5% of response for Pre-vit and Post-vit, respectively; n=4), there was no significant difference between the treatments (Figure 5B).
Figure 5: Chick biventer cervicis preparation, under no field stimulation. Exogenous ACh (A) and KCl (B) added at the end of experiment (120 min) show a responsive contracture of neutralization assays. V. haenkeana extract totally inhibited the venom effect in Pre (Pre-venom incubation treatment) to ACh addition, but partially against the Post (Post-venom incubation treatment). When KCl was added in post venom model, the responses of two models were similar. * p<0.05 compared to control. # p<0.05 compared to venom.
When preincubated with the venom Vh-E also totally preserved the contracture responses to ACh. This effect constitutes evidence that this extract totally abolished the venom action on “extrinsic”, but not on “intrinsic” nicotinic receptors (those elicited by indirect stimuli). Clearing, biventer cervicis muscle contains both focally (sensitive to electric stimulation resulting in twitch responses) and multiply (sensitive to agonist addition resulting in contracture responses) innervated fibers which, in turn, possess “intrinsic” and “extrinsic” nicotinic receptors, respectively [10,28]. According to Ginsborg [29], the nerve stimulation leads to contract the both types of muscle fibers, while exogenous ACh causes contraction of only the diffusely-innervated muscle fibers. It is believed that intrinsic receptors are less easily accessible than the extrinsic ones, due to a diffusion barrier in the narrow synaptic cleft. When the extract was added after the beginning of B. jararaca venom action, the protective effect of Vh-E was only of 55±5%.
Actions of drugs directly on muscle contractility can be assessed on preparations stimulated directly by addition of KCl or by electrical stimulation after abolition of neuromuscular transmission [10]. It is known that KCl causes muscle membrane depolarisation by releasing Ca2+ from its storage site in the sarcoplasmic reticulum (SR), which consequently activates the sarcomere [30]. In this study, we choose the first protocol. The effect of muscle fibers visualized by the contracture responses to exogenous KCl addition showed a partial protection exerted by Vh-E in both, preincubation and post venom treatments. At the same time shows the effect of the venom abolishing totally the contracture response to KCl and the level of venom influence on the muscle contractility. Besides, the biventer cervicis preparation was recommended by Harvey et al. [31], as a standard preparation for the screening of snake venoms for both, neurotoxic and myotoxic effects; and for checking that antivenom can neutralize such effects of venoms [32]. The measure of KCl contracture before and after the experiment is an indicator of muscle membrane integrity, which, in case of Vh-E showed to protect statistically against myotoxicity of B. jararaca venom.
CONCLUSION
The Vh-E neutralizes partially the neuromuscular blockade in Pre-vit, an effect that can be related to preserved function of “extrinsic” post-synaptic receptors, by measured contractures in response to ACh. The myotoxicity of the venom was significantly reduced by Vh-E in both, Pre-vit and Post-vit, by measured contractures in response to KCl.
FINANCIAL SUPPORT
This work was supported by Programa de Suporte à Pós-Graduação de Instituições de Ensino Particulares of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (PROSUP-CAPES), Programa Institucional de Bolsas de Iniciação Científica of the Universidade de Sorocaba (PROBIC-UNISO) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 04/09705-8; 07/53883-6; 08/52643-4; 12/08271-0; 15/01420-9).
REFERENCES
- WHO World Health Organization. Snake antivenoms. Fact sheet No 337. Reviewed February 2015. Ref.: https://goo.gl/ZeqZyB
- Oliveira LS, Muzitano MF, Coutinho MAS, Giany Oliveira de Melo, Sônia Soares Costa. Plantas medicinais como recurso terapêutico em comunidade do entorno da reserva biológica do Tinguá, RJ, Brasil - metabólitos secundários e aspectos farmacológicos. Revista Científica Internacional. 2011; 4: 54-74. Ref.: https://goo.gl/s6pc2z
- Indriunas A, Aoyama EM. Plantas empregadas em acidentes ofídicos de Systema Materiae Medicae Vegetabilis Brasiliensis de Martius. II Simpósio sobre a biodiversidade da Mata Atlântica. 2013; 189-194.
- Moura VM, Mourão RHV, Santos MCD. Acidentes ofídicos na região norte do Brasil e o uso de espécies vegetais como tratamento alternativo e complementar à soroterapia. Scientia Amazonia. 2015; 4: 73-84.
- Werner AC, Ferraz MC, Yoshida EH, Tribuiani N, Gautuz JA, et al. The facilitatory effect of Casearia sylvestris Sw. (guaçatonga) fractions on the contractile activity of mammalian and avian neuromuscular apparatus. Current Pharmaceutical Biotechnology. 2015; 16: 468-481. Ref.: https://goo.gl/kt9SuX
- Costa RB, Contini AZ, Melo ESP. Reproductive system of Anadenanthera peregrina and Vochysia haenkiana in a fragment of "Cerrado forest" from Chapada dos Guimarães - MT, Brazil. Ciência Rural. 2003; 33: 305-310. Ref.: https://goo.gl/62N3UP
- Cezari EJ. Plantas medicinais: atividade antitumoral do extrato bruto de sete plantas do cerrado e o uso por povos tradicionais. [Dissertação]. Universidade Federal do Tocantins. Palmas. 2010.
- Collaço RdeC, Cogo JC, Rodrigues-Simioni L, Rocha T, Oshima-Franco Y, et al. Protection by Mikania laevigata (guaco) extract against the toxicity of Philodryas olfersii snake venom. Toxicon. 2012; 60: 614-622. Ref.: https://goo.gl/hCZnda
- Ginsborg BL, Warriner J. The isolated chick biventer cervicis nerve-muscle preparation. British Journal of Pharmacology and Chemotherapy. 1960; 15. 410-411. Ref.: https://goo.gl/TYB3pU
- Harvey AL, and Marshall IG. Skeletal muscle. In: Sturkie’s avian physiology (Wittow GC ed.), 5th ed, San Diego London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press. 2000; 8: 123-139.
- Santos MG, Lolis SF, Dal Belo CA. Levantamentos etnobotânicos realizados em duas comunidades de remanescentes de negros da região do Jalapão, Estado do Tocantins. In: Pires AICS, Oliveira R. (orgs.) Sociabilidades negras. Comunidades remanescentes, escravidão e cultura. Belo Horizonte: Daliana Ltda. 2006.
- Lima PC, dos Santos MG, Calabrese KS, et al. Evaluation of the leishmanicidal activity of plant species of the Brazilian savana. Revista de Patologia Tropical. 2015; 44: 45-55.
- Amaral CFS, Silva OA, Godoy P, Miranda D. Renal cortical necrosis following Bothrops jararaca and B. jararacussu snakebite. Toxicon. 1985; 23: 877-885. Ref.: https://goo.gl/UFJfJV
- Oshima-Franco Y, Alves CMV, Andréo Filho N, Gerenutti M, Cintra ACO, et al. Neutralization of the neuromuscular activity of bothropstoxin-I, a myotoxin from Bothrops jararacussu snake venom, by a hydroalcoholic extract of Casearia sylvestris Sw. (guaçatonga). Journal of Venomous Animals and Toxins Including Tropical Diseases. 2005; 11: 465-478. Ref.: https://goo.gl/PbvGDW
- Cintra-Francischinelli M, Silva MG, Andréo-Filho N, N. Andréo-Filho, M. Gerenutti, et al. Antibothropic action of Casearia sylvestris Sw. (Flacourtiaceae) extracts. Phytotherapy Research. 2008; 22: 784-790. Ref.: https://goo.gl/zm1pMg
- Farrapo NM, Silva GAA, Costa KN, Magali Silva G, José Cogo C, et al. Inhibition of Bothrops jararacussu venom activities by Plathymenia reticulata Benth extracts. Journal of Venom Research. 2011; 2: 52-58. Ref.: https://goo.gl/JTyJ83
- Melo RS, Farrapo NM, Rocha DS, et al. Antiophidian mechanisms of medicinal plants. In: Keller RB (Ed.), Flavonoids: Biosynthesis, Biological Effects and Dietary Sources. Nova Science Publishers. 2009; 8: 249-262. Ref.: https://goo.gl/dv1BPe
- Nazato VS, Rubem-Mauro L, Vieira NAG, Rocha-Junior Ddos S, Silva MG, et al. In vitro antiophidian properties of Dipteryx alata Vogel bark extracts. Molecules. 2010; 15: 5956-5970. Ref.: https://goo.gl/s3jTwq
- Rosa LJR, Amaral Filho J, Silva MG, Magali Glauzer Silva, José Carlos Cogo, et al. The inhibitory effect of Camellia sinensis extracts against the neuromuscular blockade of Crotalus durissus terrificus venom. Journal of Venom Research. 2010; 1: 1-7. Ref.: https://goo.gl/doM89N
- Oshima-Franco Y, Rosa LJR, Silva GAA, et al. Antibothropic action of Camellia sinensis extract against the neuromuscular blockade by Bothrops jararacussu snake venom and its main toxin, bothropstoxin-I. In: Luca Gallelli (Ed). Pharmacology. Intech. 2012.
- Dal Belo DA, Lucho AP, Vinadé L, Rocha L, Seibert França H, et al. In vitro antiophidian mechanisms of Hypericum brasiliense Choisy standardized extract: quercetin-dependent neuroprotection. Biomed Research International. 2013; 2013: 943520. Ref.: https://goo.gl/XR5ybj
- Tribuiani N, Silva AM, Ferraz MC, Silva MG, Bentes AP, et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complementary and Alternative Medicine. 2014; 14: 48. Ref.: https://goo.gl/9bqdMt
- Camargo TM, Nazato VS, Cogo JC, Cogo JC, Groppo FC, et al. Bothrops jararacussu venom-induced neuromuscular blockade inhibited by Casearia gossypiosperma Briquet hydroalcoholic extract. Journal of Venomous Animals and Toxins including Tropical Diseases. 2010; 16: 432-441. Ref.: https://goo.gl/WB6rCi
- Soares-Silva JO, Oliveira JL, Cogo JC, et al. Pharmacological evaluation of hexane fraction of Casearia gossypiosperma Briquet: antivenom potentiality. Journal of Life Sciences. 2014; 8: 306-315.
- Ferreira-Rodrigues SC, Rodrigues CM, Dos Santos MG, Jean Antonio Abraham Gautuz, Magali Glauzer Silva, et al. Anti-inflammatory and antibothropic properties of Jatropha elliptica, a plant from Brazilian cerrado biome. Advanced Pharmaceutical Bulletin. 2016; 6: 573-579. Ref.: https://goo.gl/zcgZjZ
- Vatampour H. Effects of black scorpion Androctonus crasicuda venom on striated muscle preparation in vitro. Iranian Journal of Pharmaceutical Research. 2003; 2: 17-22. Ref.: https://goo.gl/7rhfxf
- Zamunér SR, da Cruz-Höfling MA, Corrado AP, Stephen Hyslop, LéaRodrigues S. Comparison of the neurotoxic and myotoxic effects of Brazilian Bothrops venoms and their neutralization by commercial antivenom. Toxicon. 2007; 44: 259-271. Ref.: https://goo.gl/jpbkKE
- Chang CC, Tang SS. Differentiation between intrinsic and extrinsic acetylcholine receptors of the chick biventer cervicis muscle. Naunyn-Schmiedeberg's Archives of Pharmacology. 1974; 282: 379-388. Ref.: https://goo.gl/iyqpSe
- Ginsborg BL. Some properties of avian skeletal muscle fibres with multiple neuromuscular junctions. Journal of Physiology. 1960; 154: 581-598. Ref.: https://goo.gl/kdFsSW
- Nelson BR, Wu F, Liu Y, Douglas M. Anderson, John McAnally, et al. Skeletal muscle-specific t-tubule protein stac3 mediates voltage-induced Ca2+ release and contractility. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110: 11881-11886. Ref.: https://goo.gl/RzheLo
- Harvey AL, Barfaraz A, Thomson E, Faiz A, Preston S, et al. Screening of snake venoms for neurotoxic and myotoxic effects using simple in vitro preparations from rodents and chicks. Toxicon. 1994; 32: 257-265. Ref.: https://goo.gl/JzGNqc
- Barfaraz A, and Harvey AL. The use of the chick biventer cervicis preparation to assess the protective activity of six international reference antivenoms on the neuromuscular effects of snake venoms in vitro. Toxicon. 1994; 32: 267-272. Ref.: https://goo.gl/xo8hQC