More Information
Submitted: August 16, 2025 | Approved: September 10, 2025 | Published: September 11, 2025
How to cite this article: Melkie W, Bekeko Z. Unlocking Landrace Potential Through Race-specific Screening and Field-level Resistance Evaluation for Durum wheat (Triticum turgidum subsp. durum (Desf.) Stem Rust Resistance under Natural Epidemic. J Plant Sci Phytopathol. 2025; 9(3): 071-087. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001158
DOI: 10.29328/journal.jpsp.1001158
Copyright license: © 2025 Melkie W, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Accessions; Disease; Landrace; Resistance; Severity; Susceptible
Unlocking Landrace Potential Through Race-specific Screening and Field-level Resistance Evaluation for Durum wheat (Triticum turgidum subsp. durum (Desf.) Stem Rust Resistance under Natural Epidemic
Webanchi Melkie1* and Zelalem Bekeko2
and Zelalem Bekeko2
1Crop and Horticulture Biodiversity Research, Ethiopian Biodiversity Institute, P.O. Box 30726, Addis Ababa, Ethiopia
2School of Plant Science, Haramaya University, P.O. Box 138. Dire Dawa, Ethiopia
*Address for Correspondence: Webanchi Melkie, Crop and Horticulture Biodiversity Research, Ethiopian Biodiversity Institute, P.O. Box 30726, Addis Ababa, Ethiopia, Email: [email protected]
Durum wheat (Triticum turgidum subsp. durum [Desf.]) Husn is a significant global food cereal. The stem rust caused by P. graminis f.sp. Pgt can result in a yield loss of up to 100%. This study aimed to identify seedling-stage resistance of durum wheat landrace accessions to prevailing races (TTKSK, TKTTF, TRTTF, and JRCQC) of the pathogen and evaluate their performance under field conditions. A total of 34 landrace accessions were tested under controlled greenhouse and field conditions. Seedlings were inoculated at the Ambo Agricultural Research Center and assessed using the 0–4 scale. Selected accessions were further evaluated in the field at a hotspot location during the main growing season. Seedling evaluation results showed variability in genotype responses for the prevailing races. Fourteen and landrace accessions (TD7226, TD7227, TD7365, TD8489, TD3750, TD3751, TD3762, TD3764, TD8217, TD8218, TD8777, TD6309, TD6984, and TD8507) exhibited resistance (IT of 2 or below) types of infections to all four races. Some accessions displayed a vulnerable response with a score of 3 to 3. A highly significant (p < 0.001) correlation was observed between disease, plant parameters, and yield. Based on the field FRS (< 30s), CI (< 20), and AUDPC (30%) of the check variety, accessions TD3750, TD3751, TD5917, and TD8778 exhibited high partial resistance. Identifying and using these landrace accessions can be beneficial in the development of durable resistance breeding strategies for novel resistant wheat varieties. However, the effectiveness of this landrace requires further molecular investigation to identify the resistance source.
Ethiopia is Africa’s second-largest wheat producer after Egypt, and it holds the top position in SSA [1]. Wheat ranks as the second most significant cereal crop in Ethiopia after maize [2]. In 2022, global wheat productivity increased by 1.9% compared with that in 2021. Ethiopia has two economically significant wheat species: Hexaploid bread wheat (Triticum aestivum) and tetraploid durum (Triticum durum) [3,4]. Wheat, a vital industrial crop, is the primary raw material for feed mills and is integral to bread, cakes, biscuits, pasta, and macaroni. It plays a crucial role in the diets of many Ethiopians, contributing approximately 15% of calories consumed for a population exceeding 90 million [5,6].
Triticum turgidum subsp. Durum (Desf) Husn, also known as durum wheat, is a significant global food cereal, cultivated over approximately 17 million hectares with an average yield of 36 metric tons per hectare [7]. Ethiopia is the largest producer of durum wheat in Sub-Saharan Africa, utilizing about 0.6 million hectares for cultivation. Despite its substantial economic and dietary importance, Ethiopia’s average wheat yield remains low compared with that of other countries. The average yield of durum wheat in Ethiopia is only 1.3 tons per hectare [8]. This low productivity can be attributed to a range of biotic and abiotic factors, including erratic rainfall patterns, inadequate agronomic practices, poor soil fertility, insect pests, and serious plant diseases such as rusts [9]. Wheat rust is a widespread disease in and around the United States [10].
In many regions of the country’s wheat-growing area, stem rust, which is caused by *Puccinia graminis f.sp. Pgt is a major limitation on production that can result in yield reductions of up to 100% during epidemic years [11-13]. Rust diseases, particularly black and yellow rusts, are recognized as the most serious risks to wheat production and have been a major focus of research since the beginning of wheat disease studies. Rust epidemics can spread across continents due to the extensive spread of urediospores [14]. Fungi that cause wheat rust are obligate host-specific parasites that can evolve into virulent races through mutation and sexual recombination. The three types of wheat rust—leaf, stripe (yellow), and stem—have significantly contributed to yield reductions and profoundly impacted global socio-economic stability worldwide [15].
Recent studies in the country have revealed that many previously identified races of wheat rust are virulent against most currently grown wheat varieties [16]. This indicates the potential risk of resistance breakdown in Ethiopian-released wheat varieties. Resistance can manifest as a decrease in the number of lesions, a reduction in pustule size, an extension of the latent period, and a shorter sporulation period [17]. The national durum wheat program in Ethiopia employs strategies such as selecting indigenous germplasm, introducing new varieties, hybridization, and evaluating selected lines to address these disease challenges and enhance yield [18].
The durum wheat landraces of Ethiopia are promising origins of resistance to stem rust and could be effectively utilized in the breeding schemes for wheat [19]. Resistance conditioned by utilizing genes has been the most widely emphasized strategy for mitigating rust threats and reducing losses incurred [20,21]. Pyramiding or cascading of several major genes into a single cultivar is also an attractive breeding strategy for increasing resistance durability by reducing stepwise accumulation of virulence by the pathogen against each gene [20,21]. The alternative is the development and employment of cultivars carrying durable or slow-rusting resistance based on quantitatively inherited, multiple genes referred to as adult plant resistance [20]. The use of gene pyramid in the management of Pgt was more efficient than the sole application of monogenic and polygenic resistance materials, probably due to the synergistic effect of gene combination in combating the pathogen [22]. Moreover, knowledge of the prevailing races is crucial as pathogens, like Pgt, are known to evolve their virulence frequently [23]. Currently, most of the released commercial wheat varieties by the national wheat research program are frequently defeated by new races of stem rust. There is a crucial need for farmers to use wheat genotypes possessing adequate resistance to emerging new physiological races of Pgt. Ethiopian farmers prioritize various traits in wheat varieties, including grain production, disease resistance, and other important social values. However, the genetic variability of pathogens complicates their management. Utilizing a wheat variety is essential for farmers to exhibit strong resistance. It is essential to create new wheat cultivars that integrate a variety of resistance mechanisms because of the quick evolution and spread of more virulent stem rust races, the frequent failures of recently created resistant varieties, and the scarcity of long-lasting resistance sources. This calls for the exploration of new resistance sources from landraces, through screening under seedlings and field conditions. Achieving long-lasting resistance against wheat stem rust requires ongoing identification and characterization of the pathogen, and deployment of new resistance genes capable of overcoming current virulent races. Landrace accessions present a promising source for discovering resistance to be utilized in breeding programs. The resistance observed in seedlings of these landrace accessions is characterized as complete and monogenic; it is governed by a single gene and provides full protection against the pathogen. This robust form of resistance is not only effective during the seedling stage but also persists throughout all growth stages of the wheat plant, ensuring ongoing protection against potential infections. The alternative is the development and employment of cultivars carrying durable or slow-rusting resistance based on quantitatively inherited, multiple genes referred to as adult plant resistance [24]. The use of gene pyramid in the management of Pgt was more efficient than the sole application of monogenic and polygenic as resistance materials, probably due to the synergistic effect of gene combination in combating the pathogen [22]. Landrace accessions could be a potential source of resistance to be exploited in breeding programs. Therefore, this research was proposed to investigate new sources of resistance in durum [Triticum turgidum subsp. durum (Desf.) Husn.] landraces accessions to the prevailing races of Pgt in a greenhouse at the seedling stage and evaluate at natural epidemics.
Study area
Field evaluations were conducted at the Bishoftu Agricultural Research Center, Ethiopia. Bishoftu is in the East Shewa Administrative Zone of the Oromia National Regional State, 47 km southeast of Addis Ababa, at 38°57’ E longitude and 08°44’ N latitude, with an elevation of 1900 m.a.s.l. [25]. It receives an average annual rainfall of 851 mm, with an average annual minimum and maximum temperatures of 8.9 °C and 28.3 °C , respectively, and a mean annual relative humidity of 61.3% [26]. It is an internationally known hotspot area of stem rust because of its suitability for the establishment and rapid epidemics of wheat stem rust. Seedling tests were conducted in a greenhouse at the Ambo Agricultural Research Center (AARC). It is located at an elevation of 2175 meters in west Shewa, with a latitude of 8°57’58’’N and a longitude of 37°51’33’’E. The average annual rainfall is 1265.7 mm, and the average annual temperature is 27.54 °C.
Planting materials
A total of 34 durum wheat accessions collected from parts of Tigray, Amhara, Oomiya, and South Nation Nationality Peoples of Ethiopia regions by the Ethiopian Biodiversity Institute were used for this study. The accessions were selected based on the passport data from different geographical locations conserved in the genebank (Table 1). As well as the susceptible reference variety (McNair, Morocco), were utilized as planting materials from Bishoftu Agricultural Research Center (DZARC) (Table 1).
| Table 1: To each of four isolated races of Pgt, thirty-four durum Wheat lines and landrace accessions were tested, coupled with one susceptible check (McNair). | |||||||
| No | Accession | Region | Zone | District | Latitude | Longitude | Altitude (m.a.s.l) |
| 1 | TD5917 | Oromiya | Misrak Shewa | Lome | 08-42-00-N | 39-11-00-E | 2000 |
| 2 | TD7226 | SNNP | Gurage | Goro | 08-25-00-N | 37-55-00-E | 2000 |
| 3 | TD7227 | SNNP | Gurage | Goro | 08-25-00-N | 37-55-00-E | 2000 |
| 4 | TD7364 | Amara | Semen Wello | Guba Lafto | 1 1-46-00-N | 39-36-00-E | 1900 |
| 5 | TD7365 | Amara | Semen Wello | Guba Lafto | 11-44-00-N | 39-35-00-E | 1910 |
| 6 | TD8063 | Oromiya | Misrak Shewa | Ada'a Chukala | 08-40-00-N | 39-06-00-E | 2000 |
| 7 | TD8489 | Unknown | Unknown | Unknown | 15-09-00-N | 38-52-00-E | 2000 |
| 8 | TD3750 | Amhara | Mirab Gojam | Dembecha | 10 -42-00-N | 37-07-00-E | 2050 |
| 9 | TD3751 | Amhara | Mirab Gojam | Dembecha | 10-42-00-N | 37-07-00-E | 2050 |
| 10 | TD3762 | Amhara | Mirab Gojam | Dembecha | 10-34-00-N | 37-29-00-E | 2050 |
| 11 | TD3764 | Amhara | Mirab Gojam | Dembecha | 10-34-00-N | 37-29-00-E | 2050 |
| 12 | TD8211 | Amhara | Mirab Gojam | Jabi Tehnan | Unknown | unknown | 2020 |
| 13 | TD8217 | Amhara | Mirab Gojam | Jabi Tehnan | Unknown | unknown | 2020 |
| 14 | TD8218 | Amhara | Mirab Gojam | Jabi Tehnan | Unknown | unknown | 2020 |
| 15 | TD8746 | Oromiya | Mirab Wellega | Sayo | Unknown | unknown | 1900 |
| 16 | TD8777 | Oromiya | Misrak Shewa | Adea | Unknown | Unknown | 1900 |
| 17 | TD8778 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1900 |
| 18 | TD8780 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1900 |
| 19 | TD8781 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1900 |
| 20 | TD3262 | Tigray | Misrakawi | Wukro | 14-16-00-N | 39-28-00-E | 1945 |
| 21 | TD6309 | Amhara | Mirab Gojam | Bure Wemberma | Unknown | Unknown | 2020 |
| 22 | TD6984 | Oromiya | Arsi | Seru | 07-40-00-N | 40-12-00-E | 1995 |
| 23 | TD6985 | Oromiya | Arsi | Seru | 07-40-00-N | 40-12-00-E | 2000 |
| 24 | TD8117 | Tigray | Mehakelegnaw | Adwa | 14-02-00-N | 38-04-00-E | 1920 |
| 25 | TD8118 | Tigray | Mehakelegnaw | Werie Lehe | 13-04-00-N | 38-04-00-E | 1980 |
| 26 | TD8119 | Tigray | Mehakelegnaw | Werie Lehe | 14-04-00-N | 38-04-00-E | 2000 |
| 27 | TD8121 | Tigray | Mehakelegnaw | Werie Lehe | 13-04-00-N | 39-35-00-E | 1850 |
| 28 | TD8123 | Tigray | Mehakelegnaw | Werie Lehe | 13-04-00-N | 39-35-00-E | 2000 |
| 29 | TD8124 | Tigray | Mehakelegnaw | Werie Lehe | 14-02-00-N | 38-04-00-E | 2000 |
| 30 | TD8504 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1900 |
| 31 | TD8507 | Oromiya | Misrak Shewa | Lome | Unknown | Unknown | 1860 |
| 32 | TD8519 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1980 |
| 33 | TD8525 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1900 |
| 34 | TD8528 | Oromiya | Misrak Shewa | Ada'a Chukala | Unknown | Unknown | 1950 |
| 35 | Susceptible Check1 McNair Both for field and greenhouse | ||||||
| 36 | Susceptible Check2 Morocco Only for the field | ||||||
Field evaluation of durum wheat landrace accessions
A field experiment was conducted at Bishoftu Agricultural Research Center (BARC) under natural infection in the 2019/2020 main cropping season, and the 34 planting materials were collected from EBI and checks (Morocco and McNair) from BARC (Table 1). The total experimental field was 13.2 m *14 m (184.8 m2). Each plot consists of four rows (0.6 m wide) and 1.5 m long and with a spacing of 0.2 m between rows, 0.5 m between plots, and 1 m between blocks and replications. The treatment was arranged in a simple lattice design with two replications. Seeding rate, fertilization, hand weeding (three times), and other management practices were applied according to the recommendations for the area. The seeding rate of the variety was 150 kg ha-1 with the spacing of 20 cm between rows. The recommended fertilizer rate in the study area is DAP 150 kg ha-1 and Urea 100 kg ha-1.
Agronomic and yield components data
Plant height (cm): the average value of ten plants was taken randomly from two central rows, and their heights were measured at maturity.
Spike Length (SPL) (cm): the average value was ten plants randomly selected from two central rows, and their spike length was measured at maturity.
Number of kernels per spike: average value. Ten plants were randomly taken from two central rows of a plot at maturity, and the number of kernels in each spike was counted after threshing.
Thousand Kernel Weight (TKW) (g): It was done by counting and weighing 250 seeds, and the final result was multiplied by 4 to get the thousand kernel weight.
Above-ground dry biomass (t ha-1): The entire plants were harvested at maturity; their weight was measured and converted into t ha-1.
Grain yield data (t ha-1): Clean grain yield from each plot was recorded and converted into tons per hectare (t ha-1).
Harvest index (HI%): Harvest index was determined as the ratio of dry grain yield to the aboveground biological yield (biomass yield) and expressed as a percentage.
Days to 50% heading: The duration recorded when 50% or more of the plants on the plots produced heads from the date of sowing.
Days to physiological maturity: It was taken as the number of days elapsed from seedling emergence to the date when 90% of the crop stems, leaves, and floral bracts in a plot changed to light yellow color.
Disease parameters: Stem rust infection of the field evaluation were recorded at the time of disease appearance, based on a 0–4 scale as described in [27] where “0” = no visible symptoms; “;” = only necrotic/chlorotic flecks without any uredia; “1” = small uredinia surrounded by necrosis; “2” = small to medium uredia surrounded by chlorosis or necrosis; “3” = medium-sized uredia without chlorosis or necrosis; “4” = large-sized uredia without chlorosis or necrosis; “X” = random distribution of variable-sized uredia; and “+” and “_” was used when uredia were somewhat larger or smaller than normal for the infection types (ITs). Seedling ITs of 0, 1, 2, and X were generally considered resistant, whereas 3 and 4 were considered susceptible. Where, Immune (I) = 0.0, Resistance (R) = 0.2, Moderately Resistant (MR) = 0.4, Moderately Susceptible- Moderately Resistant (MRMS) = 0.6, Moderately Susceptible (MS) = 0.8, Moderately Susceptible-Susceptible (MS-S) = 0.9 and Susceptible (S) = 1.0-Cobb’s scale (28)) was used only to record the stem rust severity data.
Disease incidence (DI): The number of infected plants per plot was recorded by counting infected plants per four rows and converted to disease incidence.
Disease severity (DS): A proportion of the plant affected by the disease [28].
Final Rust Severity (FRS): Terminal stem rust severity was scored at the maturity stage of the crop [28,29]
Average Coefficient of Infection (ACI): calculated by multiplying the percentage severity by a constant for host response [30]. The ACI for each accession was computed from four severity observations, and the ACI was used for calculating AUDPC for each accession.
The percentage severity index (PSI) was calculated by using the formula [31]
Area under disease progress curve (AUDPC): Calculated by the stem rust disease severity scores taken at different times [32] method.
𝑛
𝐴𝑈𝐷𝑃𝐶 = ∑ [0.5(xi + xi + 1)] [ti + 1 − ti]
𝑖 = 1
Where, xi is the cumulative disease severity proportion at the ith observation; ti is the time after planting at the ith observation, and n is the total number of observations.
Disease progress rate (Inf-rate): The disease severity was assessed four times at seven days interval from 10 randomly pre-tagged plants in the central two rows of each plot were regressed over time and the apparent infection rates as the coefficient of the regression line, ln [X/(100-X)], where X was average coefficient infection plotted against time in days [33] was calculated for each accession as tabulated (Table 2). The disease progress rate (DPR) as a function of time was calculated from disease severity observation by using a logistic regression model [34].
| Table 2: Disease progress rate (units/day) and parameter estimate of stem rust of durum wheat genotypes evaluated under field conditions at DARC, Central Ethiopia, during the 2019/2020 main cropping season. | |||||
| Treatment | Intercept | SE of intercept | D. progress rate log/day | SE of rate | R2 (%) |
| TD8525 | -5.531 | 0.473 | 0.1118 | 0.0109 | 92.10 |
| TD8218 | -5.521 | 0.160 | 0.09900 | 0.00368 | 98.77 |
| TD8063 | -5.625 | 0.182 | 0.10082 | 0.00420 | 98.46 |
| TD8781 | -5.228 | 0.323 | 0.09948 | 0.00743 | 95.19 |
| TD8504 | -4.355 | 0.262 | 0.06122 | 0.00603 | 91.89 |
| TD7226 | -5.291 | 0.295 | 0.08912 | 0.00677 | 95.03 |
| TD8117 | -4.842 | 0.398 | 0.08490 | 0.00914 | 90.45 |
| Morocco | -5.771 | 0.435 | 0.1235 | 0.0100 | 94.39 |
| TD6984 | -5.423 | 0.256 | 0.10240 | 0.00589 | 97.10 |
| TD7364 | -4.743 | 0.265 | 0.07483 | 0.00609 | 94.34 |
| TD8746 | -4.257 | 0.325 | 0.05545 | 0.00748 | 85.72 |
| TD8118 | -5.134 | 0.329 | 0.09178 | 0.00756 | 94.21 |
| TD5917 | -4.478 | 0.185 | 0.04258 | 0.00425 | 91.70 |
| TD6985 | -5.589 | 0.229 | 0.10258 | 0.00527 | 97.68 |
| TD8119 | -5.594 | 0.386 | 0.11587 | 0.00888 | 94.95 |
| TD3750 | -3.899 | 0.197 | 0.03541 | 0.00453 | 86.98 |
| TD7365 | -4.415 | 0.217 | 0.06257 | 0.00500 | 94.53 |
| TD8121 | -6.286 | 0.439 | 0.1152 | 0.0101 | 93.50 |
| TD7227 | -4.569 | 0.312 | 0.07036 | 0.00717 | 91.38 |
| TD3751 | -4.150 | 0.236 | 0.05051 | 0.00543 | 90.47 |
| McNair | -5.980 | 0.465 | 0.1312 | 0.0107 | 94.31 |
| TD6309 | -5.215 | 0.483 | 0.1015 | 0.0111 | 90.17 |
| TD3262 | -5.148 | 0.481 | 0.1006 | 0.0111 | 90.07 |
| TD8124 | -5.437 | 0.326 | 0.10010 | 0.00748 | 95.18 |
| TD8211 | -5.656 | 0.500 | 0.1087 | 0.0115 | 90.75 |
| TD8123 | -5.651 | 0.479 | 0.1112 | 0.0110 | 91.81 |
| TD8528 | -5.781 | 0.255 | 0.10820 | 0.00587 | 97.41 |
| TD8780 | -3.970 | 0.254 | 0.04828 | 0.00585 | 88.18 |
| TD3764 | -4.913 | 0.424 | 0.08272 | 0.00975 | 88.74 |
| TD8507 | -5.6068 | 0.0841 | 0.10122 | 0.00193 | 99.67 87.38 |
| TD8778 | -3.624 | 0.127 | 0.02315 | 0.00291 | |
| TD8519 | -6.108 | 0.507 | 0.1067 | 0.0116 | 90.20 |
| TD8217 | -5.146 | 0.130 | 0.08675 | 0.00298 | 98.95 |
| TD8777 | -5.589 | 0.306 | 0.10720 | 0.00703 | 96.26 |
| TD3762 | -3.970 | 0.254 | 0.04828 | 0.00585 | 88.18 |
| TD8498 | -5.530 | 0.345 | 0.11212 | 0.00792 | 95.68 |
| Disease progress rate obtained from the regression line of severity (%) against time of disease assessment (days); SE Standard error of rate and parameter estimates (intercept), and R2 Coefficient of determination for the Logistic model. | |||||
Seedling evaluation of durum wheat landrace accessions
The seedling evaluation was done at greenhouse conditions by using a completely randomized design (CRD) with two replications. Based on their economic significance for Ethiopian wheat production [35], four prominent Pgt races, TTKSK (Ug99 race), TKTTF (Digalu race), TRTTF, and JRCQC, were used to assess seedling reaction. These races’ virulence/avirulence formulae are shown in Table 3. To obtain an adequate inoculum, the spores were multiplied by inoculating susceptible McNair varieties. Five seeds of McNair and wheat landrace accessions were pre-germinated on filter paper in a petri dish, and after three days, the germinated seeds were raised in plastic pots measuring 7 cm by 7 cm by 6 cm and filled with sand, light soil, and compost in a 1:2:1 (v/v/v) ratio. The vitality of the spores injected into the landrace accessions was determined using McNair. Each race’s spores were suspended in Soltrol 170, a light mineral oil, and diluted to 1 × 105 spores per milliliter. Then, seven-day-old seedlings (Figure 1a, Figure 2a,b), when the first leaf is completely spread out and the second leaf is emerging, inoculation of spores’ suspensions of virulent races of TKTTF, TTKSK, TRTTF, and JRCQC was separately done using atomized inoculators.
| Table 3: Virulence or a virulence formula of Puccinia graminis f. sp. Pgt isolates [36] | |||
| Race | Origin | Avirulence | Virulence |
| TTKSK | Uganda | Sr24, 36, Tmp | Sr5,6,7b, 8a, 9a, 9b, 9d, 9e, 9g,10, 11, 17, 21, 30, 31, 38, McN |
| TKTTF | Ethiopia | Sr 11, 24, 31 | Sr5,6,7b, 8a, 9a, 9b, 9d, 9e, 9g,10,17,21,30,36,38, Tmp, McN |
| TRTTF | Yemen | Sr8a, 24, 31 | Sr5, 6, 7b, 9a, 9b, 9d, 9e, 9g, 10, 11, 17, 21, 30, 36, 38, McN, Tmp |
| JRCQC | Ethiopia | Sr5,7b,8a, 36,9b,10,30, Tmp,24,31,38 | Sr21, 9e, 11, 6, 9g, 17, 9a, 9d, McN |

Download Image
Figure 1: Schematic overview of the protocols for seedling evaluation of genotypes in the greenhouse at AARC, Ethiopia; (A) Seven-day-old seedling, (B) Seedling in the dew chamber for rust infection establishment.

Download Image
Figure 2: A. Germinated Durum wheat seeds on multi-pot trays filled with substrate; B. Inoculated against dominant Puccinia graminis f.sp. Pgt after being ordered in the greenhouse at the side of Pads, and C. Recording the seedling reaction of inoculated samples, and D. Reaction of the wheat with different races, respectively.
To create ideal circumstances for infection, seedlings were moistened with tiny drops of distilled water 30 minutes after inoculation and placed in a dew chamber for 18 hours in the dark at 18 to 22 °C . They were then exposed to light for 4 hours [29,35] (Figure 1b).
Once the seedlings had dried for two hours, they were moved to glass containers in the greenhouse, where they were kept at a temperature of 18 to 25 °C and a relative humidity of 60% to 70% for a 12-hour photoperiod [37]. Infection types (IT) were recorded 14 days following inoculation using a 0-4 scale [29,35]. This is described in part 2.3.2 of this paper.
Data analysis
Seedling resistance evaluation frequency of resistant and susceptible accessions data to each dominant race was analyzed by Microsoft Excel using descriptive statistics [38]. Field experimental data were analyzed by using analysis of variance (ANOVA), SAS version 9.4 statistical software, and mean comparison. Proc GLM procedure analyses of variance [39] and means computed using Least Significant Difference (LSD) tests at 5% significance level, to examine mean statistical differences among treatments.
Seedling evaluation of durum wheat landrace accessions
A total of 34 durum wheat landrace accessions and McNair were tested and evaluated at the seedling stage in a greenhouse for their reactions to four distinct Pgt races. The McNair cultivar was a universally susceptible cultivar that was susceptible to all races found, used as a control reference to benchmark the responses observed in the landrace accessions. The accessions showed different reactions to stem rust races; TKTTF, TTKSK, TRTTF, and JRCQC, while a susceptible check, McNair, exhibited high ITs for all races between 3 to 3. It showed a high level of infection, effective inoculation, and it was possible to score ITs with accuracy [40]. The reaction of the durum wheat landrace accessions for the four races was categorized as resistant (to 2+), susceptible (3- to 3), and mixed (intermediate and susceptible) infection types in the seedling test (Table 4).
| Table 4: The reactions of durum wheat landrace accessions to four stem rust races at the seedling stage in the greenhouse. | |||||||||
| Genotype code | Species | Selection history | Infection responses to Pgt races | ||||||
| Region | Zones | Woreda | Altitude(m) | TRTTF | TKTTF | TTKSK | JRCQC | ||
| TD5917 | DW | Oromiya | Misrak Shewa | Lome | 2000 | 3 | 3- | 3- | 3- |
| TD7226 | DW | SNNP | Gurage | Goro | 2000 | 2- | 2- | 2- | ;1+ |
| TD7227 | DW | SNNP | Gurage | Goro | 2000 | ;1 | ;1+ | ;1+ | 2- |
| TD7364 | DW | Amhara | Semen Wello | Guba Lafto | 1900 | 3- | 3- | 2+ | ;1+ |
| TD7365 | DW | Amhara | Semen Wello | Guba Lafto | 1910 | 2- | 2- | ;1+ | 2- |
| TD8063 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 2000 | 3 | 3- | 2+ | 3- |
| TD8489 | DW | Unknown | Unknown | Unknown | 2000 | 2+ | 2 | 2+ | 2+ |
| TD3750 | DW | Amhara | Mirab Gojam | Dembecha | 2050 | ;1+ | 2- | 2+ | 2+ |
| TD3751 | DW | Amhara | Mirab Gojam | Dembecha | 2050 | ;1 | 2 | ;1 | 2- |
| TD3762 | DW | Amhara | Mirab Gojam | Dembecha | 2050 | ;1 | 2 | ;1 | ;1+ |
| TD3764 | DW | Amhara | Mirab Gojam | Dembecha | 2050 | ;1 | ;1 | ;1 | 2- |
| TD8211 | DW | Amhara | Mirab Gojam | Jabi Tehnan | 2020 | ; | ;1 | ;1 | 3- |
| TD8217 | DW | Amhara | Mirab Gojam | Jabi Tehnan | 2020 | ;1+ | ;1 | ;1+ | 2+ |
| TD8218 | DW | Amhara | Mirab Gojam | Jabi Tehnan | 2020 | ;1 | ;1 | ; | ;1 |
| TD8746 | DW | Oromiya | Mirab Wellega | Sayo | 1900 | 3- | 2+ | 3- | 3- |
| TD8777 | DW | Oromiya | Misrak Shewa | Adea | 1900 | ;1 | 2+ | ;1 | ;1 |
| TD8778 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1900 | 3- | 3- | 2+ | 3- |
| TD8780 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1900 | 3- | 2+ | 2+ | 2+ |
| TD8781 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1900 | 3- | 3- | 2+ | 3- |
| TD3262 | DW | Tigray | Misrakawi | Wukro | 1945 | 2+ | 2+ | ;1+ | 3- |
| TD6309 | DW | Amhara | Mirab Gojam | BureWemberma | 2020 | ;1 | ;1+ | 2 | ;1 |
| TD6984 | DW | Oromiya | Arssi | Seru | 1995 | ;1+ | 2 | ;1+ | ;1 |
| TD6985 | DW | Oromiya | Arssi | Seru | 2000 | 3- | 3- | 2+ | 3- |
| TD8117 | DW | Tigray | Mehakelegnaw | Adwa | 1920 | 3- | 2+ | 2+ | 3- |
| TD8118 | DW | Tigray | Mehakelegnaw | Werie Lehe | 1980 | 3- | 2+ | 2+ | 3- |
| TD8119 | DW | Tigray | Mehakelegnaw | Werie Lehe | 2000 | 3- | 2+ | 2+ | 2+ |
| TD8121 | DW | Tigray | Mehakelegnaw | Werie Lehe | 1850 | 2+ | 2+ | 2 | 3- |
| TD8123 | DW | Tigray | Mehakelegnaw | Werie Lehe | 2000 | 2+ | 2+ | 2+ | 3- |
| TD8124 | DW | Tigray | Mehakelegnaw | Werie Lehe | 2000 | 3- | 2 | 3- | 3- |
| TD8504 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1900 | 3- | 2 | 2+ | 2+ |
| TD8507 | DW | Oromiya | Misrak Shewa | Lome | 1860 | ;1+ | 2+ | ;1+ | 2+ |
| TD8519 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1980 | 3- | 2+ | 2- | 3- |
| TD8525 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1900 | 2+ | 2+ | 2+ | 3- |
| TD8528 | DW | Oromiya | Misrak Shewa | Ada'a Chukala | 1950 | 3- | 2+ | 2+ | 3- |
| McNair Universally Pgt susceptible host AARC | 3 | 3 | 3 | 3 | |||||
| The scale described by [29,35] with IT readings of 3 (medium-size uredinia with/without chlorosis) and 4 (large uredinia without chlorosis or necrosis) considered as compatible (susceptible), while 0 (immune or fleck), 1 (small uredinia with necrosis), and 2 (small to medium uredinia with chlorosis or necrosis) as considered incompatible (resistant). Negative (-) = smaller uredinia than the normal size, and + larger than the normal uredinia | |||||||||
All accessions displayed a differential kind of reaction for the pathotype of stem rust that was employed. Most of the accessions displayed a resistant response to 2+; only some genotypes showed a vulnerable response with a score of 3- to 3. Fourteen accessions (TD7226, TD7227, TD7365, TD8489, TD3750, TD3751, TD3762, TD3764, TD8217, TD8218, TD8777, TD6309, TD6984 and TD8507) exhibited resistance infection or incompatible reaction (;1 to 2+) against to all the four races.
The scale described by [29,35] with IT readings of 3 (medium-size uredinia with/without chlorosis) and 4 (large uredinia without chlorosis or necrosis) considered as compatible (susceptible), while 0 (immune or fleck), 1 (small uredinia with necrosis), and 2 (small to medium uredinia with chlorosis or necrosis) as considered incompatible (resistant). Negative (-) = smaller uredinia than the normal size, and + larger than the normal uredinia.
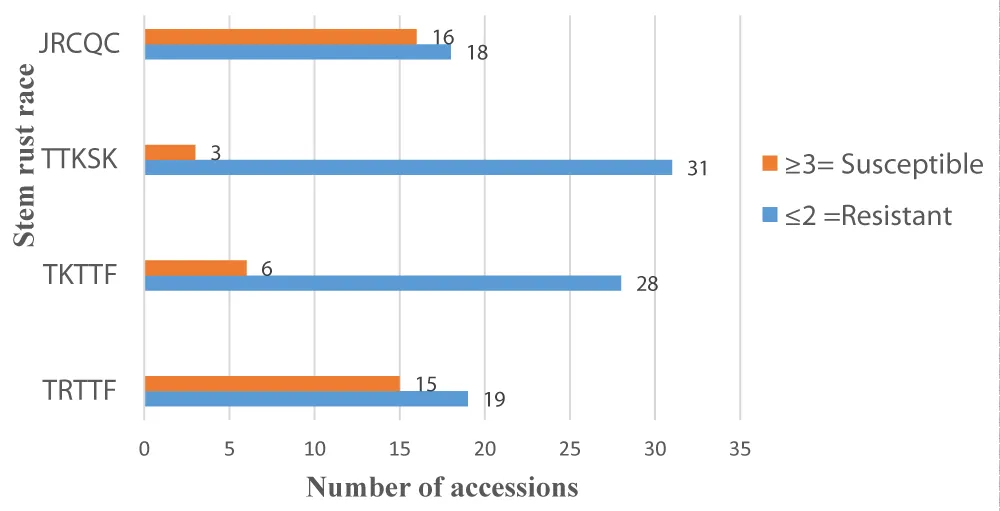
The other nineteen accessions revealed that either susceptible or resistant kind of reaction (to 3) based on the examined pathotypes, although 1 durum wheat landrace accession and McNair (susceptible check) frequently displayed a compatible kind of reaction (3 or 3-) with all pathotypes. All of the stem rust races (TTKSK, TKTTF, TRTTF, and JRCQC) displayed some degree of variability, as demonstrated in Figure 3. This result was revealed with [36] because of the presence of different avirulent genes.
Download Image
Figure 3: The frequency (number) of durum wheat landrace accessions under susceptible and resistant categories when exposed to four stem rust races (Susceptible≥ 3 and Resistance = ITs≤2.
Five landrace accessions (TD7364, TD8117, TD8118, TD8519, and TD8528) revealed resilience responses for two race combinations. The landrace accession TD7364 was incompatible with TTKSK and JRCQC; hence, it implies that they possess the Sr36 and SrTmp resistance genes. Moreover, these accessions (TD7364) may also have more unidentified resistance genes, whereas wheat accessions TD8117, TD8118, TD8519, and TD8528 were resistant to TTKSK, and they might also have unidentified resistance genes.
The two accessions, TD8746 and TD8124, showed high ITs with every pathotype, except TKTTF, the only race that is avirulent to Sr11, suggesting that Sr11 is most likely present in these accessions. The three accessions TD8780, TD811.9, and TD8504 additionally showed low ITS (2 and 2+) in all pathotypes, except the race TRTTF, with high infection. Five accessions (TD8211, TD3262, TD8121, TD8123, and TD8525) demonstrated minimal ITs for every pathotype, except JRCQC, whereas four accessions TD8063, TD8778, TD8781, and TD6985 generated high ITs for all stem rust races, except TTKSK. At this stage, the resistance of these accessions cannot be explained; nevertheless, it may be caused by the presence of another resistance gene or genes.
Field evaluation of durum wheat landrace accessions
Slow-rusting characteristics of accessions described and estimated by disease severity at a certain crop development stage, Final Rust Severity (FRS), Final Coefficient of Infection (FCI), Average Coefficient of Infection (ACI) and Area Under Disease Progress Curve (AUDPC) were used from field phenotyping data and average seedling infection type from the seedling test as criterion to identify any possible source of partial resistance to stem rust disease [41].
Disease incidence and severity: The highest (92.5%) disease incidence was recorded from the accession TD8498, while the lowest incidence was detected from TD8778 (32.5%) and TD5917 (35%) (Table 5). TD8778 and TD5917 accessions reduced disease incidence by 64.86% and 62.16%, respectively, compared with TD8498. The highest (38.0%) mean disease severity was recorded from TD8119, followed by TD8498 (36.5%). The lowest mean disease severity was observed from TD8778 (6.8%) and TD5917 (7%) (Table 5). However, it was statistically at par with TD3750 (8.8%). TD8778 and TD5917 reduced mean disease severity by 82.19% and 81.68%, respectively, as compared to TD8119.
| Table 5: Responses of durum wheat accessions for wheat stem rust mean DI (%), DS (%), FRS, FCI, ACI, PSI, AUDPC (%-days), and r-AUDPC at DZARC, during the 2019/2020 main cropping season. | |||||||||
| Genotype | DI | DS | FRS | FCI | ACI | PSI | AUDPC | rAUDPC1 | rAUDPC2 |
| TD8525 | 75.0a-g | 36.2cd | 63.0b-d | 56.7b | 31.33bc | 55.69cd | 1176b-d | 82.235b-d | 86.47b-d |
| TD8218 | 60.0f-k | 25.6g-j | 53.0d-h | 47.7b-f | 20.51e-i | 39.38g-j | 792h-l | 55.385h-l | 58.235h-l |
| TD8063 | 50.0h-m | 25.2h-k | 57.5d-f | 57.5b | 21.96d-h | 38.77h-k | 758i-l | 53.01i-l | 55.735i-l |
| TD8781 | 62.5ek | 31.0d-h | 60.0c-e | 54.0bc | 24.15d-g | 47.695d-h | 980d-h | 68.53d-h | 72.06d-h |
| TD8504 | 40.0k-m | 16.5m-o | 27.5jk | 24.75ij | 12.8j-m | 25.385m-o | 530m-o | 37.065m-o | 38.9m-o |
| TD7226 | 47.5i-m | 21.9j-m | 52.0d-h | 52.0b-e | 19.6f-i | 33.695i-m | 648k-n | 45.315k-n | 47.65k-n |
| TD8117 | 60.0f-k | 26.0g-j | 47.5f-h | 36.75f-h | 18.55f-j | 40.0gh-j | 830g-k | 58.045g-k | 61.03g-k |
| Morocco | 97.5ab | 42.0ab | 75.0a | 75.0a | 42.6a | 64.62ab | 1360ab | 95.1ab | 100ab |
| TD6984 | 62.5e-k | 30.4d-h | 59.5c-e | 50.55b-e | 23.81d-g | 46.77d-h | 958e-i | 66.99e-i | 70.44e-i |
| TD7364 | 47.5i-m | 18.0l-o | 25.0jk | 15.0j-m | 9.4lm | 27.69m-o | 600l-o | 41.96l-o | 44.12l-o |
| TD8746 | 45.0j-n | 14.5n-p | 25.0jk | 15.0j-m | 7.9mn | 22.305m-o | 460n-p | 32.165n-p | 33.825n-p |
| TD8118 | 57.5f-m | 26.5g-j | 50.0e-h | 40.0e-h | 18.1g-k | 40.77g-j | 840g-k | 58.745g-k | 61.765g-k |
| TD5917 | 35.0l-m | 7.0q | 11.5m | 4.6mn | 2.34n | 10.77q | 222qr | 15.525qr | 16.325qr |
| TD6985 | 72.5c-h | 27.3f-j | 56.5d-g | 56.5b | 22.6d-h | 42.0f-j | 846g-k | 59.16gh-k | 62.21g-k |
| TD8119 | 87.5a-d | 38.0cb | 71.0ab | 42.6c-g | 20.22e-i | 58.77bc | 1224bc | 85.595bc | 90bc |
| TD3750 | 42.5j-m | 8.8pq | 13.5lm | 5.4l-n | 2.88n | 13.54pq | 278p-r | 19.44p-r | 20.44p-r |
| TD7365 | 50.0h-m | 16.5m-o | 30.0jk | 24.0ij | 11.8k-m | 25.385m-o | 520m-o | 36.365m-o | 38.235m-o |
| TD8121 | 55.0f-m | 26.2g-j | 58.5d-f | 52.65b-d | 20.63e-i | 40.31g-j | 794h-l | 55.525h-l | 58.38h-l |
| TD7227 | 50.0h-m | 19.5l-n | 35.0ij | 31.5hi | 15.0i-l | 30.0k-n | 620l-n | 43.355l-n | 45.59l-n |
| TD3751 | 52.5g-m | 13.0op | 22.5kl | 18.0jk | 7.9mn | 20.0op | 400o-q | 27.97o-q | 29.415o-q |
| McNair | 100.0a | 44.0a | 77.5a | 77.5a | 43.4a | 67.69a | 1430a | 100a | 105.15a |
| TD6309 | 85.0a-e | 33.0c-f | 59.0de | 47.2b-f | 23.8d-g | 50.765c-f | 1064c-f | 74.405c-f | 78.235c-f |
| TD3262 | 90.0a-c | 33.8c-e | 57.5d-f | 57.5b | 27.79cd | 52.0c-e | 1102c-e | 77.065c-e | 81.03cde |
| TD8124 | 65.0d-j | 27.8e-j | 59.0de | 59.0b | 24.9d-f | 42.77e-j | 856g-k | 59.86g-k | 62.94g-k |
| TD8211 | 75.0a-g | 31.5d-g | 57.5d-f | 57.5b | 23.7d-g | 48.465d-g | 1010d-g | 70.63d-g | 74.265d-g |
| TD8123 | 92.5a-c | 33.5c-e | 60.0c-e | 51.0b-e | 26.9c-e | 51.54c-e | 1080c-f | 75.525c-f | 79.41c-f |
| TD8528 | 70.0c-i | 28.2e-i | 57.5d-f | 48.75b-f | 20.11f-i | 43.385e-i | 878f-j | 61.395f-j | 64.555f-j |
| TD8780 | 40.0k-m | 13.8n-p | 20.0k-m | 12.0k-n | 7.64mn | 21.23n-p | 452n-p | 31.61n-p | 33.235n-p |
| TD3764 | 52.5g-m | 23.3i-l | 44.0hi | 32.4g-i | 17.58g-k | 35.845i-l | 736j-l | 51.47j-l | 54.12j-l |
| TD8507 | 60.0f-k | 25.8g-j | 56.5d-g | 53.6b-d | 22.27d-h | 39.69g-j | 786h-l | 54.965h-l | 57.79h-l |
| TD8778 | 32.5m | 6.8q | 10.0m | 3.0n | 1.56n | 10.46q | 212r | 14.83r | 15.59r |
| TD8519 | 55.0f-m | 22.8i-l | 54.0d-h | 48.6b-f | 19.42f-i | 35.075i-l | 666k-m | 46.575k-m | 48.97k-m |
| TD8217 | 60.0f-k | 22.3i-m | 46.0gh | 41.4d-h | 16.38i-k | 34.305i-m | 688j-m | 48.11j-m | 50.59j-m |
| TD8777 | 77.5a-f | 31.0d-h | 62.5b-d | 44.0c-g | 18.9f-j | 47.69d-h | 970d-h | 67.835d-h | 71.325d-h |
| TD3762 | 40.0k-m | 13.8n-m | 20.0k-m | 17.0j-l | 9.84lm | 21.23n-p | 452n-p | 31.61n-p | 33.235n-p |
| TD8498 | 92.5a-c | 36.5b-d | 70.0a-c | 70.0a | 34.5b | 56.15cd | 1160c-e | 81.12c-e | 85.29c-e |
| LSD (5%) | 19.75 | 5.21 | 9.25 | 10.48 | 5.64 | 8.01 | 17.77 | 12.43 | 13.06 |
| CV% | 15.87 | 10.28 | 9.63 | 12.55 | 14.43 | 10.29 | 11.08 | 11.099 | 11.098 |
| Abbreviation: DI: Disease Incidence; DS: Disease Severity; PSI: Percent Severity Index; FRS: Final Rust Severity; FCI: Final Coefficient of Infection; ACI: Average Coefficient of Infection; AUDPC: Area Under Disease Progress Curve; r-AUDPC: Relative Area under Disease Progress Curve; r-AUDPC-1 and r-AUDPC-2: Relative Area under Disease Progress Curve with McNair and Morocco, respectively. Means in the same column followed by the same letter(s) are not statistically different at 5% level of significance according to DMRT (Duncan's Multiple Range Test). | |||||||||
Final Rust Severity (FRS): Among the accessions evaluated, 10 accessions (29.41%) showed less than 30% FRS, with field responses varying from MR to MS-S. Five accessions had severities ranging from 30 to 50%, with field responses varying from MR-MS to MS-S, while a greater number of the genotypes (19 accessions) displayed more than 50% final rust severities, which showed a susceptible type of reaction (MR-MS to S) (Appendix Table 1). Out of the 10 accessions in the first group (up to 30% FRS), TD3750, TD3751, TD3762, and TD7365 had resistance seedling reactions (to 2+); TD7364, TD8504, and TD8780 had mixed seedling reactions (1+ to 3-) while TD5917, TD8746, and TD8778 showed susceptible (3- to 3) infection types. The susceptible check, Morocco, and McNair exhibited the highest disease severity of 77.5% with a completely susceptible (S) response.
Coefficient of Infection (Cl): In this study eight accessions (TD7364, TD8746, TD5917, TD3750, TD3751, TD8780, TD8778 and TD3762) showed CI values between 0-20. These are designated as having a high level of slow-rusting. Six accessions (TD8504, TD8117, TD8118, TD73,65, TD7227, and TD3764) were under moderate levels of slow-rusting resistance (CI between 21 to 40). The other twenty accessions were grouped under low levels of slow-rusting resistance categories (CI value 40 – 60) (Table 5).
Area under the disease progress curve (AUDPC): The highest AUDPC (1430) and r-value (0.1312) were generated by the susceptible check variety, McNair, followed by Morocco (1360) with r-0.1235. Among the accessions, the highest AUDPC value was recorded from TD8119 (1224), and the lowest values were noted from TD8778 (212). Four accessions (TD5917, TD3750, TD3751, and TD8778) showed r-AUDPC values up to 30% of the check varieties McNair and Morocco. Twenty11 | Page-seven and twenty-four genotypes exhibited r-AUDPC1 and r-AUDPC2 values up to 70% of McNair and Morocco, respectively, expressing moderate slow rusting resistance, while the remaining had r-AUDPC >70% (Table 5).
Growth and yield-related components: The highest (92.05 cm) mean value for plant height was measured on the accession TD8121, while the shortest (51.9 cm) plant height was recorded from TD5917; the remaining accessions also varied from each other. TD3751 took the longest mean days to 50% flowering. TD5917 and TD8778 were ranked in the same mean height class, while the shortest duration was on TD8525, which was highly significantly different from TD3751, TD5917, TD8778, and TD8528 accessions. The longest mean day to 90% physiological maturity was recorded on TD8218, followed by TD3762. On the other hand, the shortest mean values were recorded on TD8525 accessions (Table 6). TD8525, TD8218, and TD3764 ranked in the same mean spike length class, while the shortest length was from the check variety Morocco, followed by accession TD8519. Accession TD8124 had the highest mean number of seeds per spike, followed by TD8778 and TD3751, which were statistically at par. Conversely, the lowest mean seed numbers were counted from the check variety Morocco, followed by TD 8519 (Table 6).
| Table 6: Growth, yield, and yield-related components of durum wheat accessions were evaluated for their resistance reaction against wheat stem rust under field conditions at DZARC, Central Ethiopia, during the 2019/2020 main cropping season. | |||||||||
| Genotype | DH | DTM | PH | SPL | NKPSP | TKW | BMY | GY | HI |
| TD8525 | 69.5i | 98h | 56.10h-j | 8.57a | 37.2a-d | 35.89a-e | 3.68d-g | 1.72ef | 46.83b-g |
| TD8218 | 76c-i | 125.5a | 76.60a-g | 8.5a | 35.93a-d | 27.01hg | 5.79a-f | 3.27ab | 55.5a-f |
| TD8063 | 82.5a-g | 103f-h | 58.00f-j | 7.1a-e | 35.28a-e | 38.61a-c | 3.23g | 1.91c-f | 58.49a-e |
| TD8781 | 72.5e-i | 114.0a-f | 67.20b-j | 8.4ab | 38.32ab | 34.89a-f | 3.56e-g | 1.62f | 45.36c-g |
| TD8504 | 86.5a-c | 109.5b-h | 74.10a-h | 7.14a-e | 36.67a-d | 30.97d-h | 6.0a-e | 2.56a-f | 51.26d-g |
| TD7226 | 84 a-e | 117.5 a-e | 70.95b-j | 7.93a-c | 36.57a-d | 31.89d-h | 4.95a-g | 3.11a-d | 62.2a-d |
| TD8117 | 75.5c-i | 112.5a-g | 83.65a-c | 6.33de | 31.4e-g | 26.92hg | 6.41a-c | 2.54a-f | 39.78f-h |
| Morocco | 72f-i | 116.1a-f | 67.20b-j | 5.97e | 27.44g | 27.32f-h | 3.45fg | 1.46f | 42.96d-h |
| TD6984 | 71.5g-i | 109.5b-h | 78.80a-e | 7.07a-e | 34.85a-e | 32.12b-h | 6.17a-d | 2.5a-f | 41.86e-h |
| TD7364 | 83.5a-f | 113a-g | 74.50a-h | 6.37de | 34.28a-e | 30.67f-h | 4.61a-g | 2.00b-f | 42.04e-h |
| TD8746 | 84a-e | 100gh | 82.90a-d | 7.9a-c | 36.22a-d | 29.64f-h | 6.47a-c | 3.28ab | 51.76a-g |
| TD8118 | 84.5a-d | 113.5a-f | 63.70d-j | 8.2ab | 37.33a-c | 39.9a | 4.61a-g | 2.67a-f | 57.74a-f |
| TD5917 | 90.5a | 113a-g | 51.90j | 7.4a-e | 35.82a-e | 39.66ab | 4.33c-g | 2.52a-f | 58.24a-e |
| TD6985 | 76 c-i | 109.5b-h | 57.50f-j | 7.07a-e | 34.1c-e | 36.68a-e | 4.06c-g | 2.34a-f | 57.41a-f |
| TD8119 | 74e-i | 111b-h | 78.50a-e | 7.27a-e | 33.3c-f | 27.82f-h | 7.11a | 2.34a-f | 32.55gh |
| TD3750 | 84.5a-d | 108c-h | 77.15a-f | 7.84a-d | 36.94a-d | 37.72a-d | 6.16a-d | 2.95a-e | 48.32a-h |
| TD7365 | 83a-g | 114a-f | 57.65f-j | 7.9a-c | 36.75a-d | 31.29c-h | 5.17a-g | 2.61a-f | 50.4a-g |
| TD8121 | 77c-i | 108c-h | 92.05a | 7.77a-d | 36.55a-d | 27.3f-g | 7.05ab | 2.33a-f | 33.09gh |
| TD7227 | 86 a-c | 111.5b-g | 72.40a-h | 8.17ab | 37.43a-c | 40.23a | 4.11c-g | 1.5f | 39.13e-h |
| TD3751 | 92a | 111.5b-g | 67.95b-j | 8.34ab | 37.75a-c | 36.01a-e | 4.67a-g | 2.67a-f | 57.68a-f |
| McNair | 70.5hi | 116.5a-f | 63.55d-j | 6.5c-e | 32.67d-f | 25.49h | 4.44c-f | 1.72fg | 38.75f-h |
| TD6309 | 75c-i | 110.6b-h | 78.75a-e | 8.07ab | 37.42a-c | 31.66c-h | 6.06a-e | 3.11a-d | 51.11a-g |
| TD3262 | 72f-i | 109.5b-h | 73.85a-h | 8.47ab | 37.25a-d | 30.93d-h | 5.0a-g | 2.42a-f | 47.67b-h |
| TD8124 | 80.5a-i | 114.4a-f | 62.15e-j | 8.3ab | 38.88a | 31.57c-h | 4.56b-f | 3.0a-e | 66.2ab |
| TD8211 | 72f-i | 105.5d-f | 73.35a-h | 7.47a-e | 34.84a-e | 34.12a-g | 5.20a-g | 2.39a-f | 45.67c-g |
| TD8123 | 72f-i | 120.5a-c | 84.00ab | 6.97c-e | 34.66a-e | 25.32h | 6.12a-d | 1.84d-f | 30.35h |
| TD8528 | 71.5g-i | 112b-g | 69.10b-j | 7.8a-d | 37.24a-d | 29.62e-h | 5.12a-g | 2.45a-f | 47.44b-g |
| TD8780 | 88ab | 109c-h | 63.95d-j | 7.74a-d | 37a-d | 27.81f-h | 4.17c-g | 2.39a-f | 57.4a-f |
| TD3764 | 83a-g | 119a-d | 54.65h-j | 8.5a | 37.1a-d | 29.23e-h | 5.61a-g | 3.55a | 63.5a-c |
| TD8507 | 83a-g | 117.5a-e | 66.65b-j | 7.97a-c | 35.65a-e | 27.74f-h | 5.28a-g | 1.72ef | 32.9gh |
| TD8778 | 90.5 a | 118.1a-e | 52.65ij | 7.97a-c | 38.54ab | 35.77a-e | 5.45a-g | 3.59a | 65.88ab |
| TD8519 | 80.5a-h | 118.5a-d | 70.70b-j | 6.0e | 32.55gf | 34.78a-f | 5.39a-g | 2.56a-f | 47.26b-g |
| TD8217 | 78.5b-h | 119a-d | 56.65h-j | 7.77a-d | 36.83a-d | 40.71a | 5.89a-f | 3.06a-d | 51.32a-g |
| TD8777 | 71.5g-i | 104.5e-h | 52.55ij | 8.14ab | 37.49a-c | 31.09c-h | 5.56a-g | 3.17a-c | 57.26a-f |
| TD3762 | 81.5a-h | 122.5ab | 56.95g-j | 8.23ab | 37.17a-d | 37.62a-d | 4.34c-g | 2.93a-e | 67.23a |
| TD8498 | 72.5e-i | 118.5a-d | 68.15b-j | 8.3ab | 37.1a-d | 36.18a-e | 3.67d-f | 1.59f | 43.24d-g |
| Lsd (5%) | 9.54 | 12.34 | 15.89 | 1.41 | 4.21 | 6.84 | 2.07 | 1.12 | 15.27 |
| Cv% | 5.94 | 4.88 | 11.85 | 8.14 | 5.22 | 9.50 | 18.64 | 20.09 | 13.75 |
| a) DH Days to 50% heading (days); DTM Days to 90% physiological maturity (days); PH Plant height (cm); SPL Spike length (cm); NKPSP Number of kernels per spike; TKW thousand kernel weight (g); BMY Biomass yield; GY Grain yield (t ha−1); and HI harvest index. Means in the same column followed by the same letter(s) are not significantly different at 5% level of significance according to DMRT. | |||||||||
Grain yield: The result of the evaluation revealed that very highly negative correlation between yield and the stem rust disease parameter. About a 58.22% yield gap was recorded between the resistant (TD8778) and the susceptible accession (TD7227). The highest (3.59 t ha−1) mean grain yield was obtained from TD8778, TD 3764 statistically at par; which was not significantly different from the mean grain yield obtained from TD8746 and TD8218. The lowest (1.5 t ha−1) seed yield was recorded from the accession TD7227 which had significant grain yield reduction among tested accessions next to the check variety Morocco during the cropping season (Table 6). The final disease severity recorded on accessions TD8218 was 53% but the yield obtained from the accessions was higher than some accessions such as TD8504 and TD7227, that had low disease severities.
Among the slow-rusting accessions identified, TD8778 had the highest (3.59 t ha-1) grain yield. The yields obtained from some of local wheat accessions, such as TD8781, TD8498, and TD7227, were below the yield of the susceptible variety McNair. Heavier (40.71 g) thousand seed weight was obtained from the accession TD8777 than others. But the lowest (25.32 g) seed weight was harvested from the accession TD8123 in the cropping season (Table 6).
Disease Progress Rate (DPR): Disease progress rates for the accessions ranged from 0.02315 to 0.11587 units/day. However, infection rates of all accessions were less than both Morocco and McNair. McNair had the highest (0.1312 units/day) disease progress rate than Morocco (0.1235 units/day) and landrace accessions. Most of the (18 accessions) had lower apparent infection rates, less than < 0.10 units/day (Table 2). From the accessions, the highest (0.11587 units day-1) disease progress rate was computed from TD8119, followed by TD8121 (0.1152 units day-1) and TD8498 (0.11212 units day-1). On the other hand, the lowest (0.02315 units day-1) disease progress rate was calculated from TD8778, followed by TD3750 (0.03541 units day-1) and TD5917 (0.04258 units day-1). As compared to Morocco and McNair (susceptible check), TD8119, TD8121, and TD8498 had the highest disease development. On the contrary, the accessions TD8778, TD3750, and TD5917 had the lowest disease development. Accession TD8778 had the least disease progress rate compared to the whole treatments that were used or tested.
Correlation among slow-rusting parameters, thousand kernel weight, and disease parameters
The associations among disease parameters (DI, DS, FRS, FCI, ACI, PSI, and AUDPC), growth, yield, and yield-related components were examined using simple correlation analyses. Variable levels of relationships found among disease, growth, and yield parameters (Table 7). In this study, a high and strong positive correlation at the p < 0.0001 level of significance was noted among all the epidemiological parameters: FRS, FCI, ACI, PSI, and AUDPC, which were used to assess partial resistance (Table 7). These epidemiological parameters give a dependable rate of disease increase and are related to components of partial resistance, like low receptivity, longer latent period, and smaller pustules. The correlations among the field-based slow-rusting parameters are positive and highly significantly correlated with correlation coefficients ranging from 0.801 to 1. Association results showed a very strong positive correlation (r = 0.999999. **** nearly approximate (r = 1****) and very highly significant (p < 0.0001) level of association between disease severity and AUDPC values (Table 7). AUDPC becomes directly proportional to final disease severity, resulting in an approximately linear relation [42]. Both disease incidence and severity had positive and very highly significant (p < 0.0001) correlations with AUDPC, CI, and FRS. The high correlation coefficient was also computed between AUDPC and final rust severity.
| Table 7: Coefficients of correlation (r) among disease, growth, and yield parameters of durum wheat accessions at Bishoftu Agricultural Research Center, during the 2019 main cropping season. | ||||||||||||||||
| Parameters | DH | DTM | PH | SPL | NKPSP | TKW | BMY | GY | HI | DI | DS | FRS | FCI | ACI | PSI | AUDPC |
| DH | 1 | |||||||||||||||
| DTM | 0.10* | 1 | ||||||||||||||
| PH | 0.23* | 0.10* | 1 | |||||||||||||
| SPL | 0.13 | -0.01 | -0.231 | 1 | ||||||||||||
| NKPSP | 0.29 | 0.08* | 0.232* | 0.875**** | 1 | |||||||||||
| TKW | 0.36 | -0.112 | 0.469*** | 0.271 | 0.340* | 1 | ||||||||||
| BMY | -0.04 | 0.015 | 0.583*** | -0.056 | -0.019 | -0.409* | 1 | |||||||||
| GY | 0.35* | 0.14 | -0.127 | 0.329* | 0.356* | 0.047* | 0.508** | 1 | ||||||||
| HI | 0.45*** | 0.112 | 0.638**** | 0.404** | 0.417* | 0.423** | --0.305* | 0.652**** | 1 | |||||||
| DI | 0.83**** | -0.001 | 0.171 | -0.156 | -0.348* | -0.370* | -0.068* | -0.433** | -0.456** | 1 | ||||||
| DS | -0.88**** | -0.038 | 0.182 | -0.173 | -0.369* | -0.394* | -0.101* | -0.485** | -0.471** | 0.929**** | 1 | |||||
| FRS | -0.83**** | 0.001 | 0.169 | -0.154 | -0.352* | -0.351* | -0.071 | -0.424** | -0.417* | 0.851**** | 0.961**** | 1 | ||||
| FRC | -0.77**** | 0.049 | 0.112 | -0.148 | -0.336* | -0.266 | -0.23 | -0.480** | -0.345* | 0.801**** | 0.907**** | 0.957**** | 1 | |||
| ACI | -0.79**** | 0.054 | 0.104 | -0.205 | -0.406* | -0.347* | -0.268 | -0.546*** | -0.400** | 0.869**** | 0.944**** | 0.92**** | 0.953**** | 1 | ||
| PSI | -0.88**** | -0.038 | 0.182 | -0.173 | -0.369* | -0.394* | -0.101 | -0.485** | -0.471** | 0.929**** | 1**** | 0.961**** | 0.907**** | 0.944**** | 1 | |
| AUDPC | -0.88**** | -0.048 | 0.182 | -0.174 | -0.367* | -0.399* | -0.108 | -0.495** | -0.479** | 0.936**** | 0.998**** | 0.939**** | 0.884**** | 0.939**** | 0.0.997**** | 1 |
| DH: Days to 50% Heading (days); DTM: Days to 90% physiological maturity (days); PH : Plant Height (cm); SPL: Spike Length (cm); NKPSP: Number of Kernel Per Spike; TKW: Thousand Kernels Weight (g); BMY: Biomass Yield(t ha-1); GY: Grain Yield (t ha−1 ); HI: Harves index; DI: Disease Incidence; DS: Disease Severity; PSI: Percent Severity Index; FRS: Final Rust Severity; FCI: Final Coefficient of Infection; ACI: Average coefficient of Infection and AUDPC: Area Under Disease Progress Curve; **** Correlations is very highly significant at <0.0001 level. *** Correlations are highly significant at the 0.001 level. ** Correlation is significant at the 0.01 level. *Correlation is significant at the 0.05 level. | ||||||||||||||||
Plant height had a significant and positive correlation with days to 50% heading, days to 90% physiological maturity, spike length, the number of kernels per spike, and thousand kernel weight. Days to 50% heading showed a significant and positive correlation with days to 90% physiological maturity. Number of kernels per spike had a significant (p < 0.05) and positive correlation (r = 0.356*) with grain yield and a significant positive correlation (r = 0.340*) with thousand kernel weight. The grain yield increases when the number of kernels per spike increases. The correlation coefficients considered between pairs of the respective disease parameters (DI, DS, FRS, FCI, ACI, PSI, and AUDPC) and TKW were highly and negatively correlated. When the disease epidemiology increases, the plant pathogen affects the rate of photosynthesis and affects the nutrient supply of the host. This can affect reducing TKW, NKPSP, and the total above-ground biomass of the genotypes. Days to 50% heading have a highly significant (p < 0.0001) negative correlation with disease slow rusting parameters. The negative highly correlated (r = -0.88, p < 0.0001) of AUDPC and DH showed that early heading accessions tend to have higher late heading accessions show lower disease progression. A very strong correlation of DH and AUDPC, DS, DI, PSI, FRS, and ACI reinforces that the early heading is associated with more disease or favors disease development. Grain yield slightly positive correlation (r = 0.35*, p < 0.05) with DH, revealing that later heading tends to yield more due to a longer growth period or reduced disease exposure. On the other hand, higher HI increases yield (r = 0.45*, p < 0.05). A very strong positive correlation between SPL and NKPSP (r = 0.875*****, p < 0.001), HI and PH (r = 0.638, p < 0.001) indicates that longer spike gives more kernels and taller plants have better resources. AUDPC is very highly correlated with the disease parameter used as an integrated measure of disease progress [42]. Higher disease pressure significantly reduces grain yield, harvest index, and other yield components. This revealed that stem rust epidemics reduce photosynthetic area, causing significant loss [43]. The strongest impact is on GY and HI, which are direct indicators of plant productivity. Disease pressure is not strongly influenced by plant parameters. AUDPC negatively correlates with TKW because disease reduces grain filling through assimilated translocation disturbance [44].
The identification of resistance traits of durum wheat landrace accessions, by exposing them to various Puccinia graminis tritici pathotypes, assesses the presence and effectiveness of resistance genes through seedling testing. The gene-for-gene theory is a fundamental concept that explains how specific resistance in plants, such as durum wheat accessions, is determined by the interaction between host resistance genes and pathogen avirulence genes. There is a corresponding avirulence gene in the pathogen for every resistance gene found in the host plant (Table 2). The interaction leads to a race-specific resistance mechanism, where the effectiveness of the plant’s defense response depends upon the compatibility of these genetic components.
The compatible reaction of durum wheat landrace accessions (TD5917) and McNair indicates that it does not have any major gene or has fewer effective genes against the examined pathotypes. This result agrees with [45] and [40]; a high IT on a tested accession means that it lacked any of the resistance genes for the examined pathotype. However, these accessions may contain resistance genes to the races not included in this test or under natural settings. Because the TD5917 accession is resistant to natural epidemics.
Based on the result, incompatible reaction of 14 landrace accessions (TD7226, TD7227, TD7365, TD8489, TD3750, TD3751, TD3762, TD3764, TD8217, TD8218, TD8777, TD6309, TD6984 and TD8507) to different races may possess identified resistance genes and may also have more unidentified resistance genes, because these accessions either have other resistance genes that are not yet recognized or most likely carry the resistance gene Sr24, which is effective against all tested races or might have any of the genes, either alone or in combination, for which the test pathotypes exhibited avirulence [45]. This result revealed that [46] and [47] suggested that the presence of two or more minor genes may imply multiple (horizontal) disease resistance, or the presence of an effective main R-gene against several races may be indicated by an incompatible response to two or more races.
Most of the accessions used in this greenhouse investigation showed low compatible infection reactions. The seedling evaluation result revealed some varying degrees of accessions variability in responses to the TTKSK, TKTTF, TRTTF, and JRCQC races. This may be the presence of an effective main R-gene against several races [47].
All the races employed in this investigation demonstrate variation in reactivity among test genotypes, with the majority of the genotypes exhibiting resistance reaction with scores ranging from 0 to 2+ (Figure 2). Identifying these sources of resistance and creating resistant cultivars are essential in relation to stem rust outbreaks. Seedling resistance to stem rust is often conferred by specific resistance (R) genes. These genes can provide effective resistance against various races of the pathogen. Notable R genes include Sr31, Sr24, and Sr36, which have been widely studied and utilized in breeding programs. The results of this evaluation (Table 4) highlight the diversity of resistance among the landrace accessions and also serve as a valuable resource for breeding programs aimed at enhancing disease resistance in durum wheat. This finding confirms that Ethiopian durum wheat landrace accessions have high levels of stem rust resistance [48].
Disease parameters, yield, and yield-related components data showed significant variation among treatments. Typical characteristic symptoms of the rust first appeared on check varieties. The mean rust severity on durum accessions revealed different levels of damage. Analysis of variance showed that there were highly significant (p < 0.0001) differences among the tested accessions for disease incidence and disease severity (Table 5). This result revealed that genetic diversity among accessions can lead to varying levels of resistance to diseases [49].
There was a wide variation in the stem rust mean FRS, ranging from 10 to 77.5% during the cropping season (Table 4). Diverse field reactions ranging from Resistant (R) to susceptible (S) responses were observed. Analysis of variance showed that there were highly significant (p < 0.0001) differences among tested accessions for final rust severity. There was considerable variation in the final rust severities of the accessions tested, which might be due to differences in the number of resistance genes present and the mode of gene action. Wheat accessions with FRS values of 1%–30%, 31%–50% and 51%–70% were regarded as possessing high, moderate, and low levels of slow rusting resistance, respectively [50]. Accessions (TD3750, TD3751, TD3762 and TD7365, TD7364, TD8504, TD8780, TD5917, TD8746 and TD8778) with a low FRS (1%-30%) under high disease pressure may possess more additive genes or genes with larger effects [51]. FRS represents the cumulative result of all resistance factors during the progress of epidemics. [52] and [21] also used final severity as a parameter to assess the slow rusting behaviour of wheat.
Some of the accessions showed low to moderate severity (< 20) at natural epidemics, and this would give a chance to identify valuable accessions for future breeding and pathological research. From the seedling and field response against stem rust of wheat at natural epidemics, three accessions (TD3750, TD3751, and 3762) showed resistance for both the seedling and adult-plant stages (Tables 4 and 5). Possibly, these accessions might have all-stage resistance [53]. Accessions (TD7226, TD7227, TD8489, TD3764, TD8217, TD8218, TD8777, TD6309, TD6984, and TD8507) possessed seedling resistance (Table 4), but failed to protect at the adult-plant stage (Table 5). The seedling resistance is not growth stage-dependent, does not always protect against rust at adult-plant stages [53]. A genotype resistance at the seedling stage alone is not sustainable and effective for long-term deployment [44]. Often, seedling resistance is governed by major gene(s), and frequent mutations in corresponding avirulence genes in the rust pathogen may lead to catastrophic failure of the crop [54].
Three accessions, TD5917, TD8746, and TD8778, showed susceptible seedling reaction but resistance to natural epidemics. However, three accessions (TD7364, TD8780, and TD8504) showed intermediate seedling reaction and field-resistant reaction at natural epidemics. Field resistance is often effective against a wide range of pathogen races and considered more durable, providing resistance without being readily overcome by the change in pathogen virulence when the cultivar is widely grown in an area where the disease is prevalent [55]. The deployment of cultivars carrying APR based on multiple genes is particularly preferred to delay infection, growth, and reproduction of the pathogen in adult plants and circumvent “boom-and-bust” cycles [56].
Based on host plant and pathogen interaction, there was a highly significant (p < 0.0001) difference in the CI value. Eight accessions (TD7364, TD8746, TD5917, TD3750, TD3751, TD8780, TD8778, and TD3762) showed CI values between 0-20, designated as having a high and good level of slow-rusting (Table 5). Six accessions (TD8504, TD8117, TD8118, TD7365, TD7227, and TD3764) showed moderate levels of slow-rusting resistance, which means CI value between 21 to 40 categories; the other twenty accessions were grouped under low levels of slow-rusting resistance categories (CI value 40 - 60) [57]. Slow-rusting resistance to wheat stem rust using the coefficient of infection expresses the presence of different partial resistance conferring genes in wheat accessions [58-60].
According to ANOVA results, there was a highly significant (p < 0.0001) difference in AUDPC. According to [61-63], AUDPC is a good indicator of adult plant resistance under field conditions. It is directly related to yield loss according to [64] for each 1 percent increase in AUDPC, there is a corresponding 1.8–2.0 kg/ha drop in grain yield, and provides critical information for designing effective disease management practices for accessions with different levels of resistance [42]. Accession TD5917, TD3750, TD3751, and TD8778, which had low AUDPC and terminal severity values may have high level of field resistance [32] These Accession had AUDPC up to 30% of the check varieties and had MR to MS types of infection in the field and were considered to have good levels of partial resistance and expressing good levels of slow- rusting. This revealed that accession with variable field infection responses of MR-MS to S are expected to possess genes that confer partial resistance [65,66]. Therefore, selection of an accession having low AUDPC with terminal disease score is normally accepted for practical purposes where slow rusting resistance is utilized as one of the slow resistances [67,68].
According to analysis of variance (ANOVA), days of 50% heading, days to 90% physiological maturity, plant height, spike length, number of kernels per spike, and thousand kernel weight were highly significant (p < 0.001) for the tested accessions. Biomass yield and harvest index were significantly (p < 0.05) varied (Table 6). Grain yield and TKW were highly significant (p < 0.001) differences among the tested accessions. From the outset, it should be emphasized that the differences in grain yield among the entries could be explained not only by differences in the levels of disease attack, but also in the yield potential of the varieties. Stem rust reduces the grain yields of wheat cultivars [69-71]. Thus, the best accession for grain yield was selected and advanced to the next stage of evaluation. Even if accession TD8121 had the highest plant height and high spike length, the yield was not high because the FRS (58.5) and AUDPC (794) were high, i.e, the accession was highly infested with rust. The lowest (1.5 t ha−1) seed yield was recorded from the accession TD7227, which had a significant grain yield reduction among the tested accessions. The yield from heavily rusted plants is, therefore, much reduced, and the quality of the grain is lowered, and the grains would shrivel. The effect of rust on grain yield is due to the great injury to the photosynthetic surface of the plant [72,73] and the energy expenditure in plant defence mechanisms rather than for growth and grain formation [43] According to [74] rust infection lowers leaf water potential and turgor in both infected and adjacent uninfected tissues, highlighting impairment of water relations even under well-watered conditions. Also, [75] and [76] suggested that the fungus also reduces the food and water supply within the plants. The fungus needs food and water for spore production that would otherwise be used in the formation of well-developed kernels. Further, there is a loss of water by evaporation through the numerous ruptures caused by the fungal pustules. The final disease severity recorded on accession TD8218 was 53% but the yield obtained from the accession was higher than some accessions, such as TD8504 and TD7227, that had low disease severities. These revealed that accessions that have high disease severity recorded gave higher yield than some accessions that had low disease severity [52]. Accession (TD8778) that had comparatively better yield (3.59 t ha-1) makes it a superior candidate as a gene donor parent for the incorporation of durable resistance into the durum wheat improvement programme. The yields obtained from accessions TD8781, TD8498, and TD7227 were below McNair due to their lower genetic potential for yield. Although there were variations in grain yields among the entries, there was no protected check plot established for each accession to obtain information to calculate yield loss (Table 6).
Disease development showed significantly different rates of progression. Accession TD8778 had the least disease progress rate (0.023) compared to the whole treatments that were evaluated. Infection rate showed more variation among the tested accessions than disease severity and AUDPC, and it did not distinguish accessions displaying different levels of slow-rusting resistance regarding other parameters. For example, the accession TD5917 had FRS, CI, and r-AUDPC less than the accession TD3750, but its infection rate was higher (0.425) (Table 7). These results were in agreement with the stem rust and leaf rust of wheat [57,77-79] infection rate should be used in combination with other disease parameters.
A very high and strong positive correlation was noted among all the epidemiological parameters: FRS, FCI, ACI, PSI, and AUDPC, which were used to assess partial resistance at p < 0.001. These epidemiological parameters give a dependable rate of disease increase and are related to components of partial resistance, like low receptivity, longer latent period, and smaller pustules [80]. Association results showed a very strong positive correlation (r = 1****) and a very highly significant (p < 0.0001) association between disease severity and AUDPC values. The high correlation coefficient was also computed between AUDPC and final rust severity(r = 0.939****). This implies that there is an increase in disease parameters. This finding was in agreement with [52] and [81], who found that severity and AUDPC have the highest and very strong positive correlation. Severity and AUDPC also had positive and very highly significant (p < 0.0001) correlations with disease incidence. The positive correlations among the parameters observed are in agreement with the results of other researchers on cereal rust patho-systems [78,79,82]. All disease parameters were highly correlated in the present study, suggesting that FRS and CI are considered as preferable selection parameters or criteria. There were strong negative correlations (r = -0.424** and-0.546**) between final severity, coefficient of infection) and grain yield, respectively. This implies that when there is an increase in disease parameters, there is a decrease in yield parameters and vice versa. The overall results of the correlation analysis suggest a strong negative association between stem rust and the yield component. TD8778 had a low AUDPC (212) (Table 5) and a low coefficient of infection. It has a good level of resistance [50,83,84] reported higher selection gains of slow rusting resistance using low final ratings CI and AUDPC under field conditions.
The correlation coefficients considered between pairs of the respective disease parameters (DI, DS, FRS, FCI, ACI, PSI, and AUDPC) and TKW were highly and negatively correlated (Table 7). The negative relationship between TKW and disease parameters showed the harmful effects of stem rust on this yield component (TKW). The large negative correlations between TKW and stem rust parameters could be attributed the fungus damages vascular system of the susceptible host plant extensively limiting the transportation of water and nutrients from the soil to the developing kernel and other organs as well as interfering with translocation of photosynthate, which leads to shrivelled grains [84-86]. Further, the present study detected high correlations for infection parameters and yield variables suggesting that the ranking of the wheat accession for these variables did not change significantly over time. Several other studies also concluded that disease and yield parameters have negative associations [87-89] for various reasons and could result in recognizable yield reductions.
Based on the above investigation of the coefficient of infection (0-20) and AUDPC TD8778, TD5917, TD3750, TD3751, TD3762, TD8746, and D7364 accessions had better field resistance and performance. Accessions TD3750, TD3751 3751 and TD3762 were resistant to the prevailing race at the seedling stage. [90,91] suggested that when genotypes show rust resistance at both seedling and adult plant stages, it can be referred to as all-stage resistance. However, accession TD5917 was susceptible to all races, and TD8778 was susceptible to three race combinations at the seedling stage. The deployment of accession carrying adult plant resistance based on multiple genes is particularly preferred to delay infection, growth, and reproduction of the pathogen in adult plants and circumvent “boom-and-bust” cycles [70]. Based on FRS (< 30s), CI (< 20), and AUDPC (30%) of the check variety, the accessions TD3750, TD3751, TD5917, and TD8778 exhibited a high level of partial resistance. While accession TD3762, TD7365, TD8746, TD7364, TD8504, TD8780 had FRS (< 30s), CI (< 20), and AUDPC above30% and 70% of the chick variety expressing moderate slow rusting resistance. The remaining accessions have a low level of resistance.
A high level of variability in responses of accessions to the prevailing races (TTKSK, TKTTF, TRTTF, and JRCQC) and the majority (fourteen landrace accessions) of the accessions showed resistance reaction (to 2+). All races were positive and highly correlated with each other’s i.e, have some common virulence and avirulence genes for the pathogens. From the field experiments, there was phenotypic variation of infection types and level of stem rust severity for wheat accessions with terminal scores ranging from 10 (MR) to 75 S (highly susceptible). Between the check and accessions, there are highly significant (p < 0.0001) differences among different disease parameters (DI, DS, FRS, FCI, AUDPC, and ACI) and TKW. The greater number of accessions were grouped under the MR and S types of reaction to final rust. Landrace accessions TD3750, TD3751, and 3762 showed both seedling and adult-plant stages resistance at natural epidemics, which can be referred to as all-stage resistance. However, three accessions, TD5917, TD8746, and TD8778, showed seedling susceptibility but adult plant resistance at natural epidemics.
Correlation coefficients for slow rusting parameters were positive and highly significant (p < 0.0001). Yield parameters also had a positive correlation among themselves. The disease parameters maintained a negative and highly significant relationship with yield traits. Grain yield had a highly significant (p < 0.001) and positive correlation with the number of kernels per spike and a significant positive correlation with thousand kernel weight. However, slow rusting parameters and TKW were highly and negatively correlated.
In conclusion, developing novel resistant wheat cultivars may benefit from the use of these accessions in breeding operations. By pyramiding several stem rust resistance genes, it is crucial to increase the genetic base of stem rust resistance in future wheat cultivars. The effectiveness of these landrace accessions found in the current study, which also includes other Sr genes, requires additional molecular investigation in order to determine the cause of their resistance and apply it to wheat breeding initiatives. Based on field and greenhouse evaluation, accessions TD3750, TD3751, TD5917, and TD8778 exhibited a high level of partial resistance. Of these, TD5917 and TD8778 have true slow rusting resistance, and TD3750 and TD3751 were resistant to prevailing races in the greenhouse and have a good level of field resistance. So, they have all-stage resistance. Among the slow-rusting accessions, comparatively better TKW and grain yields were produced by TD5917 (39.66 g) and TD8778 (3.59 t ha-1), respectively. The slow-rusting accession (TD3750, TD3751, TD5917, and TD8778) identified from this study can be used for durable stem rust resistance breeding. Such Ethiopian durum wheat landraces accessions accessions are an important source to develop resistant cultivars for rust disease outbreaks and other major diseases. Further study is required across locations for the compatibility study and factors contributing to the disease epidemic for early warning, and to assess the association of wheat stem rust intensity and yield loss of the identified accessions with the comparison of protected standard check. Molecular investigation is necessary to identify an effective source of genes for their resistance.
The authors would like to express their large gratitude to the Ethiopian Biodiversity Institute for their support. The authors also extend many thanks to the Ambo Agricultural Research Center Wheat Rust Research Team for providing the greenhouse facilities and technical assistance throughout the duration of the research.
Availability of data
The article includes the data that was utilized to support the study’s conclusions.
Contributions of the authors’
The authors made meaningful contributions to this study in data collection and editing of the manuscript.
- FAO STAT. Crops and Livestock Products. FAO Statistics. 2022. Available from: https://www.fao.org/faostat/en/#data/QC.
- Central Statistical Agency. Agricultural Sample Survey 2020-21. Volume I: Report on Area and Production of Major Crops. Addis Ababa. 2021. Available from: https://ess.gov.et/wp-content/uploads/2013/09/2013EC.LAND-UTILIZATION-2021.pdf
- Belayneh A, LVFWOF. Virulence analysis of Puccinia graminis f. sp. Pgt populations in Ethiopia with special consideration of Ug99. Plant Pathol. 2009; 58(2):362–9. Available from: https://doi.org/10.1111/j.1365-3059.2008.01976.x
- Effect of lime and vermicompost amendments on selected soil properties and wheat (Triticum aestivum L.) productivity on Nitisols of Negasa area, East Wollega Zone, Ethiopia. Available from: http://ir.haramaya.edu.et/hru/handle/123456789/2060
- FAO. Crop Prospects and Food Situation - Quarterly Global Report No. 1, March 2022. 2019.
- Minot N, Warner J, Lemma S, Kasa L, Gashaw A, Rashid S. The wheat supply chain in Ethiopia: Patterns, trends, and policy options. Gates Open Res. 2019; 3(174). Available from: https://doi.org/10.21955/gatesopenres.1115226.1
- Royo C. Durum wheat: A global perspective. Agronomy. 2016; 6(3):54.
- FAO. The spread of damaging wheat rust continuous new races found in Europe, Africa, and Central Asia. FAO Report. 2017; 40
- C. Relationship between stripe rust (Puccinia striiformis) and common wheat (Triticum aestivum) yield loss in the highlands of Bale, southeastern Ethiopia. Arch Phytopathol Plant Prot. 2009; 42(6):508–23. Available from: http://dx.doi.org/10.1080/03235400701191663
- Kolmer A, Ordonez ME, Groth JV. The Rust Fungi. In: Encyclopedia of Life Sciences. 2009; 18. Available from: https://www.ars.usda.gov/ARSUserFiles/3094/rust_fungi.pdf
- Ayele B, BH. Incidence and challenges of rusts in wheat production, in Bishaw et al. (Ed.), Containing the Menace of Wheat Rusts: Institutionalized Interventions and Impacts.
- Embete F, BAKZ. Identification of sources of resistance to stem rust of wheat, Puccinia graminis f.sp. Pgt. Report of completed research project from 1999-2004. 2005; Volume I: Pathology. Available from: PPRC, Ambo.
- Hei NB. Evaluation of wheat cultivars for slow rusting resistance to leaf rust (Puccinia Pgtna Eriks) in Ethiopia. Afr J Plant Sci. 2017; 11(23):23–9. Available from: http://dx.doi.org/10.5897/AJPS2016.1450
- Khan MH, Bukhari A, Dar ZA, Rizvi SM. Status and strategies in breeding for rust resistance in wheat. Agric Sci. 2013; 4(6):292–301. Available from: http://dx.doi.org/10.4236/as.2013.46042
- Rehman AU, Sajjad M, Profile S, Khan S, Ahmad N. Prospects of wheat breeding for durable resistance against brown, yellow, and black rust fungi. Int J Agric Biol. 2013; 15:1209–20. Available from: http://dx.doi.org/10.13140/2.1.4219.7121
- Admassu B, Lind V, Friedt W, Ordon F. Virulence analysis of Puccinia graminis f.sp. Pgt populations in Ethiopia with special consideration of Ug99. Plant Pathol. 2009; 58(2):362–9. Available from: https://doi.org/10.1111/j.1365-3059.2008.01976.x
- Roelfs AP, Saari RE, McNab A. Rust Diseases of Wheat: Concepts and Methods of Disease Management. Mexico: CIMMYT. 1992; 81.
- Zemede Lemma A. Genetic erosion, drought tolerance, and genotype by environment interaction of durum wheat (Triticum turgidum L.var durum) in Ethiopia. 2019. Available from: https://www.scribd.com/document/638051333/Untitled
- Denbel W, Badebo A. Valuable sources of resistance in the Ethiopian durum wheat landraces to UG33 and other stem rust races. Int J Agron Plant Prod. 2012; 3(6):191–5. Available from: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20123384629
- Oliver R. Achieving durable disease resistance in cereals. Burleigh Dodds Science Publishing; 2021. Available from: https://www.bspp.org.uk/achieving-durable-disease-resistance-in-cereals/
- Tilahun Hadis L, Negash Gure T, Kassa Habtemariam D, Muche Abebile G, Yirga Belayineh F, Ayele Zerihun A. Evaluation of Wheat Genotypes for a Single Stem Rust Race TTTTF in Ethiopia. Biomed Stat Inform. 2021; 6(3):47. Available from: http://dx.doi.org/10.11648/j.bsi.20210603.12
- Chen J, Ubhayasekera N, Dong Y, Singh J, Lian F, Bao C, et al. Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science. 2017; 358(6370):1607–1610. Available from: https://doi.org/10.1126/science.aao4810
- Jin Y, Szabo LJ, Fetch TJr, Pretorius ZA, Njau P. Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f.sp. Pgt. Plant Dis. 2009; 93:367–70. Available from: https://doi.org/10.1094/PDIS-93-4-0367
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, et al. Phenotypic and Genotypic Characterization of Race TKTTF of Puccinia graminis f.sp. Pgt that Caused a Wheat Stem Rust Epidemic in Southern Ethiopia. Phytopathology. 2015; 2013–4. Available from: https://doi.org/10.1094/PHYTO-11-14-0302-FI
- Leta G, Belay G, Worku W. Nitrogen Fertilization Effects on Grain Quality of Durum Wheat (Triticum turgidum L. Var. Durum) Varieties in Central Ethiopia. J Agric Sci Belihuloya. 2013; 1(1):1–7.
- Denbel W, Badebo A, Alemu T. Evaluation of Ethiopian Commercial Wheat Cultivars for Resistance to Stem Rust of Wheat Race UG99. Int J Agron Plant Prod. 2013; 4:15–24. Available from: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20133059480
- Roelfs AP, Singh RP, Saari EE. Rust Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, DF: CIMMYT. 1992. Available from: https://www.scirp.org/reference/referencespapers?referenceid=52073
- Peterson R, Campbell A, Hannah AE. A Diagrammatic Scale for Estimating Rust Intensity on Leaves and Stems of Cereals. Can J Res. 1948;26:490–500. Available from: https://doi.org/10.1139/cjr48c-033
- Stakman EC, Stewart DM, Loegering WQ. Identification of Physiologic Races of Puccinia graminis var. Pgt. Revised Edition. Miscellaneous Publication No. E-617. St. Paul, MN: U.S. Department of Agriculture, Agricultural Research Service. 1962; 53. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19631101079
- Roelfs AP. Epidemiology in North America. In: Diseases, Distribution, Epidemiology, and Control. Academic Press. 1985;403–34. Available from: https://www.sciencedirect.com/science/article/abs/pii/B9780121484026500213
- Wheeler BEJ. An Introduction to Plant Diseases. 1969;374. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19701604180
- Wilcoxson, Skovmand B, Atif AH. Evaluation of Wheat Cultivars for Ability to Retard Development of Stem Rust. Ann Appl Biol. 1975; 80(3):275–81. Available from: https://doi.org/10.1111/j.1744-7348.1975.tb01633.x
- Van der Plank JE. Plant Diseases. Elsevier Science Academic Press. 1963;349. Available from: https://books.google.co.in/books/about/Plant_Diseases.html?id=I6iWez_Rvq8C&redir_esc=y
- Madden LV, Hughes G, Van Den Bosch F. The Study of Plant Disease Epidemics. American Phytopathological Society. 2007; No. 632.3 M33. Available from: https://doi.org/10.1094/9780890545058
- Hailu E, Woldaeb G, Danbali W, Alemu W, Abebe T. Distribution of Stem Rust (Puccinia graminis f.sp. Pgt) Races in Ethiopia. Adv Crop Sci Tech. 2015; 3(3). Available from: http://dx.doi.org/10.4172/2329-8863.1000173
- Letta T. Seedling Resistance to Stem Rust (Puccinia graminis f.sp Pgt) and Molecular Marker Analysis of Resistance Genes in Some Wheat Cultivars. Plant. 2018; 6(1):16. Available from: http://dx.doi.org/10.11648/j.plant.20180601.13
- Getaneh Woldeab, EH, N. Bacha. Protocols for Race Analysis of Wheat Stem Rust (Puccinia graminis f.sp. Pgt). EIAR, Ambo, Ethiopia. 2017;1-26.Available from: www.globalrust.org/race-manual/Ambo.
- Dretzke B. Statistics with Microsoft Excel. Prentice Hall Press. 2008. Available from: https://dl.acm.org/doi/abs/10.5555/1481657
- Mohammad MJ, Mazahreh N. Changes in Soil Fertility Parameters in Response to Irrigation of Forage Crops with Secondary Treated Wastewater. Commun Soil Sci Plant Anal. 2003; 34(9-10):1281–94. Available from: https://doi.org/10.1081/CSS-120020444
- Admassu B, Lind V, Friedt W, Ordon F. Virulence Analysis of Puccinia graminis f.sp. Pgt Populations in Ethiopia with Special Consideration of Ug99. Plant Pathol. 2009;58(2):362–9. Available from: https://doi.org/10.1111/j.1365-3059.2008.01976.x
- Pathan AK, Park RF. Evaluation of seedling and adult plant resistance to stem rust in European wheat cultivars. Euphytica. 2007;155(1):87–105. Available from: https://link.springer.com/article/10.1007/s10681-006-9308-z
- Jeger MJ, Viljanen-Rollinson SLH. The use of the area under the disease-progress curve (AUDPC) to assess quantitative disease resistance in crop cultivars. Theoretical and Applied Genetics. 2001;102:32–40. Available from: https://link.springer.com/article/10.1007/s001220051615
- Singh RP, Hodson DP, Huerta-Espino J, Jin Y, Njau P, Wanyera R, et al. Will Stem Rust Destroy the World’s Wheat Crop? Advances in Agronomy. 2008;98:271–309. Available from: https://doi.org/10.1016/S0065-2113(08)00205-8
- Basnet BR, Singh S, Lopez-Vera EE, Huerta-Espino J, Bhavani S, Jin Y, et al. Molecular mapping and validation of SrND643: A new wheat gene for resistance to the stem rust pathogen Ug99 race group. Phytopathology. 2015;105(4):470–476. Available from: https://doi.org/10.1094/PHYTO-01-14-0016-R
- Yu LX, Barbier H, Rouse MN, Singh S, Singh RP, Bhavani S, et al. A consensus map for Ug99 stem rust resistance loci in wheat. Theoretical and Applied Genetics. 2014;127(7):1561–1581. Available from: https://doi.org/10.1007/s00122-014-2326-7
- Liu W, Maccaferri M, Rynearson S, Letta T, Zegeye H, Tuberosa R, et al. Novel sources of stripe rust resistance identified by genome-wide association mapping in Ethiopian durum wheat (Triticum turgidum ssp. durum). Front Plant Sci. 2017;8:774. Available from: https://doi.org/10.3389/fpls.2017.00774
- Khan MH, Bukhari A, Dar ZA, Rizvi SM. Status and strategies in breeding for rust resistance in wheat. Agricultural Sciences. 2017;4(06):292. Available from: http://dx.doi.org/10.4236/as.2013.46042
- Safavi SA, Ahari AB, Afshari F, Arzanlou M. Slow rusting resistance in Iranian barley cultivars to Puccinia striiformis f. sp. hordei. J Plant Prot Res. 2013;53(1):5–11. Available from: https://doi.org/10.2478/jppr-2013-0001
- Zhang H, Chen X. Advances in breeding for disease resistance in crops: The role of genomics. Front Plant Sci. 2017;8:1038. Available from: https://doi.org/10.3389/fpls.2017.01038
- Hei N, Shimelis HA, Laing M, Admassu B. Assessment of Ethiopian wheat lines for slow rusting resistance to stem rust of wheat caused by Puccinia graminis f. sp. Pgt. Journal of Phytopathology. 2015;163(5):353–363. Available from: https://doi.org/10.1111/jph.12329
- Prasad P, Thakur R, Bhardwaj SC, Savadi S, Gangwar OP, Lata C, et al. Virulence and genetic analysis of Puccinia graminis Pgt in the Indian sub-continent from 2016 to 2022 and evaluation of wheat varieties for stem rust resistance. Front Plant Sci. 2023;14. Available from: https://doi.org/10.3389/fpls.2023.1196808
- Singh M, Lakhran L, Kumar S, Kumar N, Prajapati S. Breeding approaches for disease resistance in crop plants: A review. Ann Clin Lab Sci. 2021;4(2):1022. Available from: https://meddocsonline.org/annals-of-biotechnology/breeding-approaches-for-disease-resistance-in-crop-plants-a-review.html
- Periyannan S, Milne RJ, Figueroa M, Lagudah ES, Dodds PN. An overview of genetic rust resistance: From broad to specific mechanisms. PLoS Pathog. 2017;13(1):e1006380. Available from: https://doi.org/10.1371/journal.ppat.1006380
- Dinglasan E, Periyannan S, Hickey LT. Harnessing adult-plant resistance genes to deploy durable disease resistance in crops. Essays in Biochemistry. 2022;66:571–580. Available from: https://doi.org/10.1042/EBC20210096
- Brown JKM. Durable resistance of crops to disease: A Darwinian perspective. Annu Rev Phytopathol. 2015;53:513–539. Available from: https://doi.org/10.1146/annurev-phyto-102313-045914
- Ali S, Shah SJA, Khalil IH, Raman H, Maqbool K, Ullah W. Partial resistance to yellow rust in introduced winter wheat germplasm in the north of Pakistan. Aust J Crop Sci. 2009;3:37–43. Available from: https://www.researchgate.net/publication/26575918_Partial_resistance_to_yellow_rust_in_introduced_winter_wheat_germplasm_at_north_of_Pakistan
- Draz IS, Abou-Elseoud MS, Kamara AEM, Alaa-Eldein OAE, El-Bebany AF. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann Agric Sci. 2015;60(1):29–39. Available from: https://doi.org/10.1016/j.aoas.2015.01.001
- Mitiku M, Bacha Hei N, Abera M. Characterization of slow rusting resistance against stem rust (Puccinia graminis f. sp. Pgt) in selected bread wheat cultivars of Ethiopia. Adv Crop Sci Technol. 2018;6(5):389. Available from: http://dx.doi.org/10.4172/2329-8863.1000389
- Shikha K, Chand R, Mishra NK, Singh S, Sayiprathap BR, Nair RM, et al. Components of slow disease development: A key to enhance resistance in crops. CABI Agric Biosci. 2024;5(81). Available from: https://doi.org/10.1186/s43170-024-00293-4
- Chu B, Yang L, Wang C, Gu Y, Yuan K, Wang R, et al. Improved evaluation of wheat cultivars (lines) on resistance to Puccinia striiformis f. sp. Pgt using molecular disease index. Plant Dis. 2019;103(6):1206–1212. Available from: https://doi.org/10.1094/PDIS-07-18-1158-RE
- Wang ZL, Li LH, He ZH, Duan XY, Zhou YL. Seedling and adult plant resistance to powdery mildew in Chinese bread wheat cultivars and lines. Plant Dis. 2005;89:457–463. Available from: https://doi.org/10.1094/PD-89-0457
- Sakr N. In vitro quantitative resistance components in wheat plants to Fusarium head blight. Open Agric J. 201927;13(1):9–18. Available from: http://dx.doi.org/10.2174/1874331501913010009
- Devi HM, Mahapatra S, Das S. Assessment of yield loss of wheat caused by spot blotch using a regression model. Indian Phytopathol. 2018;71:291–294. Available from: https://doi.org/10.1007/s42360-018-0036-9
- Parlevliet JE. Partial resistance of plants to fungal pathogens: Partial resistance, quantitative resistance, or slow rusting. Euphytica. 1988;38:1–11.
- Zhan J, Thrall PH, Papaïx J, Xie L, Burdon JJ. Playing on a pathogen’s weakness: Using evolution to guide sustainable plant disease control strategies. Annu Rev Phytopathol. 2015;53:19–43. Available from: https://doi.org/10.1146/annurev-phyto-080614-120040
- Hei NB. Evaluation of wheat cultivars for slow rusting resistance to leaf rust (Puccinia Pgtna Eriks) in Ethiopia. Afr J Plant Sci. 2017;11:23–29. Available from: http://dx.doi.org/10.5897/AJPS2016.1450
- Assefa M, Sileshi F, Bacha N, et al. Seedling resistance of selected Ethiopian bread and durum wheat lines against dominant stem rust races. J Plant Pathol. 2019;101:115–120. Available from: https://doi.org/10.1007/s42161-018-0157-0
- Pretorius ZA, Singh RP, Wagoire TS, Payne T. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. Pgt in Uganda. Plant Dis. 2000;84(2):203. Available from: https://doi.org/10.1094/PDIS.2000.84.2.203B
- Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, et al. Will stem rust destroy the world’s wheat crop? Adv Agron. 2008;98:271–309. Available from: https://doi.org/10.1016/S0065-2113(08)00205-8
- Ashmawy MA, Orabey WM, Shahin AA. Effect of stem rust infection on grain yield and yield components of some wheat cultivars in Egypt. Int J Phytopathol. 2013;2(3):171–178. Available from: https://esciencepress.net/journals/index.php/phytopath/article/view/308/236
- Bethenod O, Huber L, Slimi H. Photosynthetic response of wheat to stress induced by Puccinia recondita and post-infection drought. Photosynthetica. 2001;39:581–590. Available from: https://doi.org/10.1023/A:1015664314720
- Robert C, Bancal MO, Ney B, Lannou C. Wheat leaf photosynthesis loss due to leaf rust, with respect to lesion development and leaf nitrogen status. New Phytol. 2005 Jan;165(1):227–241. Available from: https://doi.org/10.1111/j.1469-8137.2004.01237.x
- Chen YE, Cui JM, Su YQ, Yuan S, Yuan M, Zhang HY. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front Plant Sci. 2015 Sep 28;6. Available from: https://doi.org/10.3389/fpls.2015.00779
- Fernando P. Water relations of faba bean uninfected and infected with rust: Effects on transpiration, leaf water potential, and root growth. In: Rust Fungi and Global Change. 2001.
- Craigie JH. Stem Rust of Cereals. Ottawa, ON, Canada: Department of Agriculture. 1957. Available from: https://publications.gc.ca/site/eng/9.800794/publication.html
- Rowell JB, Bushnell WR. The premature death of adult rusted wheat plants in relation to carbon dioxide evolution by the root system. Phytopathology. 1968;58:651–658. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/19681102666
- Safavi SA. Sources of resistance in advanced barley lines to Puccinia striiformis f. sp. hordei. Adv Environ Biol. 2012;6(2):708–712. Available from: https://www.researchgate.net/publication/289217652_Sources_of_resistance_in_advanced_barley_lines_to_Puccinia_striiformis_f_sp_hordei
- Safavi SA, Afshari F. Identification of resistance to Puccinia striiformis f. sp. Pgt in some elite wheat lines. J Crop Prot. 2012. Available from: https://www.researchgate.net/publication/303605019_Identification_of_resistance_to_Puccinia_striiformis_f_sp_Pgt_in_some_elite_wheat_lines
- Safavi S, Afshari F. A seven-year assessment of resistance durability to yellow rust in some wheat cultivars in Ardabil province, Iran. J Crop Prot. 2017;6(3):409–421. Available from: https://jcp.modares.ac.ir/article-3-10332-en.html
- Siricord C, O’Brien PA. MALDI-TOF mass spectrometry can be used for detection of pathogenic microorganisms in soil. Australas Plant Pathol. 2008;37:543–545. Available from: https://link.springer.com/article/10.1071/AP08052
- Adila W, Terefe H, Bekele A. Common bacterial blight (Xanthomonas axonopodis pv. phaseoli) resistance reaction in common bean genotypes and their agronomic performances in Southern Ethiopia. J Crop Sci Biotechnol. 2021;24:387–400. Available from: https://doi.org/10.1007/s12892-021-00087-4
- Phenotypic and molecular characterization of wheat for slow rusting resistance against Puccinia striiformis Westend. f.sp. Pgt. J Phytopathol. 2010;158:393–402. Available from: https://doi.org/10.1111/j.1439-0434.2009.01631.x
- Iqbal N, Eticha F, Khlestkina EK, Weidner A, Röder MS, Börner A. The use of simple sequence repeat (SSR) markers to identify and map alien segments carrying genes for effective resistance to leaf rust in bread wheat. Plant Genet Resour. 2007;5(2):100–103. Available from: https://doi.org/10.1017/S1479262107672311
- Zegeye H, Rasheed A, Makdis F, Badebo A, Ogbonnaya FC. Genome-wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLoS One. 2014;9:e105593. Available from: https://doi.org/10.1371/journal.pone.0105593
- Merkuz A, Getachew A. Impact of wheat stem rust (Puccinia graminis f. sp. Pgt) on yield and yield components of wheat in South Tigray, Northern Ethiopia. J Plant Pathol Microbiol. 2012;3:1–4.
- Ayele B, Alemayehu G, Disasa T. Impact of stem rust on grain yield and yield components of bread wheat in South Eastern Ethiopia. Afr J Agric Res. 2020;15:264–270.
- Lemessa F, Yaynu Hailu. Relationship of common rust (Puccinia sorghi Schw.) and yield of maize in South-western Ethiopia. East Afr J Sci. 2011;5:57–65.
- Boersma JG. Impact of leaf rust on wheat yield: Genotype, environment, and management interactions. Crop Prot. 2015;67:127–139.
- Shewaye Y, Zegaye H, Tadesse Z, Solomon T, Asnake D, Alemu G, et al. Impact of stem and yellow rusts on grain yield of bread wheat (Triticum aestivum L.) genotypes under rainfed conditions of Ethiopia. Int J Bioorganic Chem. 2021;6(1):7. Available from: http://dx.doi.org/10.11648/j.ijbc.20210601.13
- Park RF. Stem rust of wheat in Australia. Aust J Agric Res. 1994;45(6):1–10.
- Cheng P, X. M, Campbell KG. Stripe rust resistance in wheat: Genes, genetic mapping, and breeding applications. Front Plant Sci. 2022;13:832316.