More Information
Submitted: August 19, 2025 | Approved: August 26, 2025 | Published: August 27, 2025
How to cite this article: Akbar GW, Hojaifa U, Ismail T, Bristy NN, Chowdhury T, Absar N. Comparative Analysis of Physicochemical Properties and Bioactive Compounds in Two Hilly Region Pineapple Varieties (Ananas comosus L.) for Value-Added Food Applications. J Plant Sci Phytopathol. 2025; 9(2): 062-070. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001157
DOI: 10.29328/journal.jpsp.1001157
Copyright license: © 2025 Akbar GW, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pineapple; Bromelain; Proteolytic activity; Partial precipitation; Medical applications
Comparative Analysis of Physicochemical Properties and Bioactive Compounds in Two Hilly Region Pineapple Varieties (Ananas comosus L.) for Value-Added Food Applications
Gazi Wafa Akbar* , Ummay Hojaifa, Tania Ismail, Noshin Nower Bristy, Tuhina Chowdhury and Nurul Absar
, Ummay Hojaifa, Tania Ismail, Noshin Nower Bristy, Tuhina Chowdhury and Nurul Absar
Department of Biochemistry and Biotechnology, University of Science and Technology Chittagong, Chattogram – 4202, Bangladesh
*Address for Correspondence: Gazi Wafa Akbar, Department of Biochemistry and Biotechnology, University of Science and Technology Chittagong, Chattogram – 4202, Bangladesh, Email: [email protected]
Bromelain is a crude protein extract rich in proteolytic enzymes, derived from various parts of the pineapple (Ananas comosus), a member of the Bromeliaceae family. It has numerous uses in biotechnology and medicine, such as meat tenderization, digestive aid, and anti-inflammatory properties. This study aimed to extract, estimate, and partially purify bromelain from two pineapple varieties collected from hilly regions of Bangladesh (Rangamati and Bandarban), and to evaluate their physicochemical and biochemical characteristics alongside the proteolytic activity of bromelain derived from pulp and peel. Two local pineapple types, Honey Queen (HQ) and Giant Kew (GK), were gathered. Pulp and peel were homogenized in 0.1M phosphate buffer (pH 7.0), filtered, and centrifuged to create crude bromelain extract. Lowry’s technique was used to determine the protein concentration. Titrimetric (Gelatin Digestion Unit, GDU) and spectrophotometric (azo-casein hydrolysis) techniques were used to measure proteolytic activity. The optimal temperature and pH were determined. Precipitation with ammonium sulphate was used to partially purify the extract. The fruits’ biochemical and physicochemical characteristics were also examined. The highest GDU-based proteolytic activity was found in GK pulp, while azo-casein hydrolysis showed the highest activity in GK peel and the lowest in GK pulp. Bromelain was enzymatically active within a pH range of 5.0–8.0 and a temperature range of 35.5–70 °C. The ammonium sulfate precipitation method successfully concentrated the enzyme without significant loss of activity. Physicochemical evaluation revealed that HQ had superior nutritional and biochemical properties compared to GK. According to the study, pineapples grown in mountainous areas show potential as a source of bromelain. The enzyme maintains its functional stability across a broad pH and temperature range owing to the efficient extraction and partial purification procedure, making it appropriate for several industrial and medicinal applications.
Bromelain, the proteolytic enzyme, is present in the tissues of plants in the Bromeliaceae family, of which pineapple (Ananas comosus) is a well-known source. It is dispersed in varying amounts throughout the fruit’s many sections. Pineapple is among the world’s most significant commercial fruit crops. Its exceptional flavor and taste have earned it the title of “queen of fruits” [1,2].
A major contributor to human consumption, nutrition, and medicine, pineapple ranks fourth in Bangladesh in terms of total planting area and production (BBS, 2014) [2,3]. Although the fruit is produced in around 90 nations worldwide, Bangladeshi pineapples are more juicy and delicious than those from other countries, so the future of pineapple cultivation in this nation is bright. In general, it is planted practically everywhere in Bangladesh, particularly in high, hilly areas where water does not stagnate. Despite not being a tropical nation, several regions of Bangladesh have soils and a climate that are far more conducive to pineapple cultivation. [4]. Tangail, Mymensingh, Gazipur, Sylhet, Moulvibazar, Chittagong, Bandarban, Khagrachari, and Rangamati districts are among those that cultivate it extensively [5,6].
At least 90 varieties of pineapple are cultivated worldwide. However, Bangladesh mainly grows three kinds of pineapple. The three types are Red Spanish (Ghorashal), Honey Queen (Jaldubi), and Giant Kew (kalendar in the area) [7]. The Chattogram hilly zone, Rangamati, and Bandarbon are where the majority of the types of Honey Queen and Giant Kew are grown.
In addition to being consumed naturally, pineapple is processed to produce canned slices, chunks, smash, and juice. This procedure produces wastes such as peel, stem, and centrifuged solids (from juice manufacturing), accounting for 35–40% of the pineapple’s bulk [6,8]. Due to their low commercial value, these by-products are either processed to create bran for cattle feed or returned to the fields as soil amendment. The enzyme bromelain is abundant in these wastes, which can be collected and purified and can be used to determine their proteolytic activity and other nutritional contents. Furthermore, recent research has demonstrated that bromelain has numerous advantages across a range of corporations, including the culinary, pharmaceutical, medical, and cosmetic sectors. Bangladesh, one of the world’s largest pineapple-producing countries, generates a significant amount of waste that is not properly disposed of. As a result, our nation has a rare potential to produce huge amounts of bromelain from the stem, peels, and crown, which can then be used for therapeutic purposes [9].
The main purpose of this study was to evaluate the amount of bromelain present in two pineapple types that were collected from Bangladesh’s hilly regions (Rangamati and Bandarban), as well as assess their physicochemical and biochemical properties and the proteolytic activity of pulp and peel-derived bromelain.
Study area
The study was conducted as part of a thesis project from 2017 to 2018. The samples were collected from two hilly regions of Bangladesh – Rangmati and Bandarbon, in September and October of 2017, and all the biochemical tests were carried out in the University of Science and Technology Chittagong (USTC) laboratory.
Collection of the sample
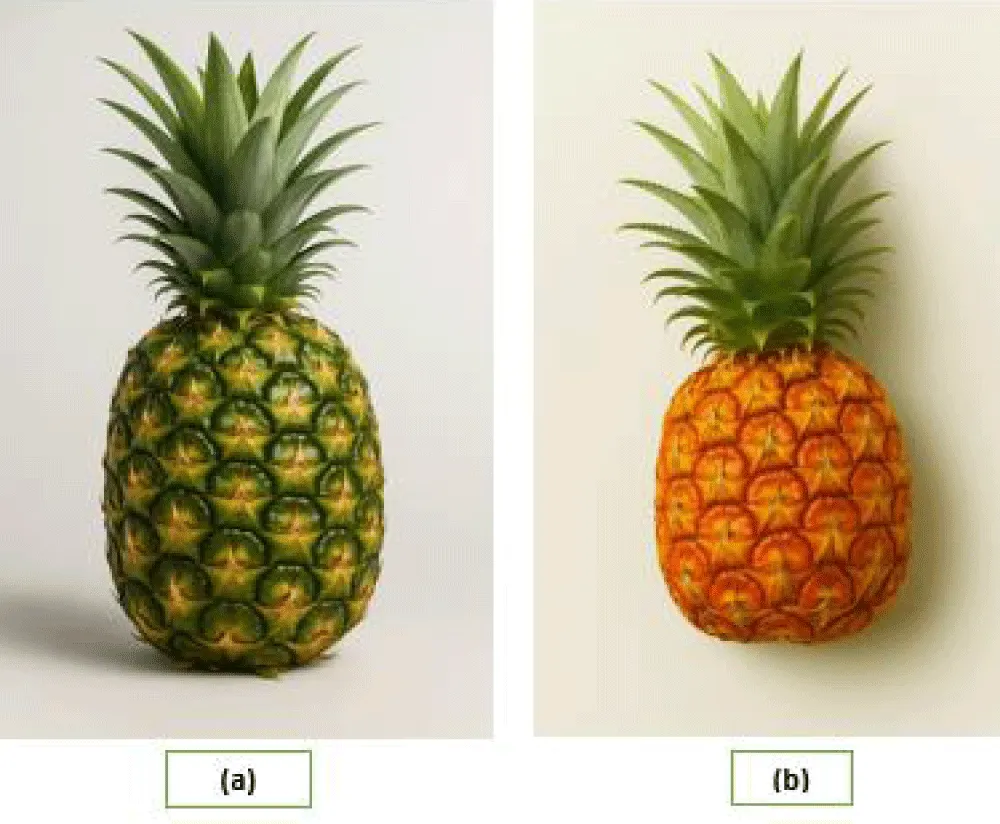
The two varieties of Pineapple, namely “Honey Queen” denoted as “HQ” or local name “Jaldubi”, and “Giant Kew” denoted as “GK”, locally known as “Jum Kew” or simply “Kew, were collected from local markets of Bandarban and Rangamati (Figure 1).
Download Image
Figure 1: Two distinct varieties of pineapple — (a) Giant Kew (GK) and (b) Honey Queen (HQ) – collected from the Bandarban region of Bangladesh. The figure represents the morphological differences between the two varieties. The Giant Kew variety appears larger with a more cylindrical shape and dark green skin, while the Honey Queen is smaller, more tapered, and exhibits an orangish-yellow color.
Collection of Giant Kew (GK): The Giant Kew pineapple, denoted as “GK”, locally referred to as “Jum Kew”, is one of the predominant commercial pineapple varieties cultivated in the Bandarban and Rangamati districts of the Chittagong Hill Tracts (CHT) in Bangladesh. The huge dimensions, cylindrical shape, and low fibre content of its delicious, juicy flesh make this variety popular. Because of its durability and long shelf life, it is especially well-suited for export and transportation industries.
Collection of Honey Queen (HQ): The Honey Queen pineapple, denoted as “HQ”, locally known as “Jaldubi”, is grown in the districts of Bandarban and Rangamati in Bangladesh’s Chittagong Hill Tracts. This type, which is well-known for its smaller size, sweetness, soft texture, and attractive appearance, has grown to be an essential part of these districts’ agricultural environment. Even if it is of excellent quality, early harvests driven by growth regulators and a lack of storage facilities frequently result in market surplus and financial losses, as per the report of The Daily Star. (2020). Pineapple chips: A saviour for farmers in the hills.
Sample and juice preparation
The samples were washed and rinsed with running tap water to remove any dust particles repeatedly. Pineapples were weighed using an electronic balance. The length, diameter, and edible portion of the samples were measured using a measuring tape. The weight of the fruit with and without the crown was weighed and recorded.
The pineapple was divided into pulp and peel, and caution was maintained for the pulp part during removal.
The pulp and peel are weighed separately, then the pineapple is sliced into small pieces using a sterile knife, as shown in Figure 2: Two Pineapple varieties processed for analysis, while wearing gloves, and the juice is extracted using a hand juicer. A sterile blender was used to blend the extracted pulp and juice. To get a clear juice, the homogenate was manually cleared using a sterile cotton cloth.

Download Image
Figure 2: Two Pineapple varieties processed for analysis — The pulp and peel parts of two pineapple types, Giant Kew (GK) and Honey Queen (HQ), were processed for biochemical and enzymatic analysis. This included determining the sugar content, protein concentration, protease activity, and other physicochemical parameters.
The processed juice was then kept in the refrigerator for experimental studies.
Physicochemical analysis
Determination of pH: At first, 2g of pineapple pulp and peel were thoroughly mixed with 30ml of distilled water and then filtered through Whatman’s No. 1 filter paper. The clear supernatant was then collected after the filtrate was centrifuged for 10 minutes at 4000 rpm. By putting the pH electrode into the filtrate and taking three suitable readings, the pH of the extracted solution was measured.
Determination of Total Soluble Solids (TSS): TSS was determined by the Refractometer as degree Brix (°B). At first, 2 g of the edible part of the pineapple was taken into a mortar and smashed well. After that, a drop of pineapple juice was dripped onto the Abbe refractometer’s prism, and the percentage of TSS derived from the device’s direct reading was noted.
Determination of Titratable Acidity (TA): The TA is the total amount of acid present in the juice and is determined by titration using a standard solution of sodium hydroxide, which can be expressed as an equivalence of any organic acid, such as citric acid, malic acid, etc [10].
In a blender, 10 grams of pineapple pulp were mixed with distilled water in a 100 ml beaker. After filtering, the combined components were taken to a volumetric flask for titration. Then 10 ml of pulp solution was taken, and 2-3 drops of phenolphthalein indicator were added, and then titrated against 0.1N NaOH solution until a persistent pink hue developed.
The total acidity can then be calculated using the following formula [10]:
% acid = T x M x milli-equivalent factor / Volume
Determination of moisture content: The traditional method was used to determine the moisture content. At first, 2g of the pineapple’s edible portion was weighed in a porcelain crucible and boiled to 100 °C for almost six hours in an electrical oven. After cooling in a desiccator, it was weighed once again. The crucible containing the sample was heated repeatedly and cooled before being weighed to maintain a constant weight.
Percent moisture content (g/100g sample) = Weight of moisture x 100/Weight of sample taken [11].
Determination of dry matter content: The percent dry matter content of the pulp was calculated from the data obtained during moisture estimation using the following formula:
Percentage of dry matter = 100 - Percentage of moisture content [11]
Biochemical and phytochemical analysis
Determination of total sugar content: The total sugar content of the pineapple’s edible section was calculated using Anthrone’s method [12].
Determination of reducing sugar content: Reducing sugar content of pineapple juice was determined by the Dinitro-salicylic acid method [12]
Determination of non-reducing sugar: The non-reducing sugar present in the sample was determined from the values of total sugar and reducing sugar. Sucrose content was calculated from the following formula [12].
Percent of non-reducing sugar = (% of Total sugar - % of Reducing sugar)
Determination of water-soluble protein content: Using bovine serum albumin (BSA) as a standard, the Folin-Lowry method [13] was used to determine the water-soluble protein content in pineapple juice.
Determination of lipid content: Lipid content of the pineapple juice was determined by the method of Bligh and Dyer [14].
Detection of phenolic compound presence: The presence of total phenolics in pineapple was determined according to the Folin-Ciocalteu [15] procedure.
A 1/10 dilution of Folin-Ciocalteu’s reagent in water was added to a test tube containing 0.2 mL of the diluted sample extract. The tubes were then allowed to stand at room temperature for 30 minutes while being shaken occasionally to allow for the development of colour; the presence of phenols in pineapple juice was detected by the colour change.
Detection of flavonoid presence: The presence of total flavonoid content of pineapple extract was determined by the aluminum chloride colorimetric method [16]. A 1:1 mixture of 1 ml of sample and 1 ml of methanolic AlCl3.6H2O solution was prepared. Additionally, distilled water and methanolic solution were combined in a 1:1 ratio to make a blank solution. Both the mixtures were incubated for 20 minutes at room temperature in a dark place. The presence of flavonoid molecules was shown by the colour change.
Determination of Vitamin C: The vitamin C content of pineapple juice was determined by the dichlorophenol indophenol (DCPIP) method [17]. In a 100 mL conical flask, 5 mL of pineapple solution was first mixed with 10 mL of 4% oxalic acid. After that, the mixture was titrated with the DCPIP solution (5 x 10-4 mol L-1) until a pink colour formed, which persisted for 30 seconds.
Extraction of bromelain and enzymatic assay
Crude extraction of bromelain: After being washed with distilled water, the pineapple fruit was dried and peeled. Figure 2 illustrates how the pineapple peel and pulp were processed. Using a blender, 10g of pulp and peel were combined with 30ml of cold phosphate buffer (pH 7.0, 0.1M). The mixture was filtered with Whatman filter paper (150 mm) or cheesecloth. The filtrate was centrifuged at 4000 rpm for 10 minutes to remove insoluble materials. Finally, the supernatant was collected and referred to as ‘Crude bromelain extract’ [18], and was used for further experiments.
Bromelain protease activity using the azo-casein method: The protease activity of bromelain was determined using the azo-casein method [19]. In this assay, 0.2 mL of bromelain solution was mixed with 0.2 mL of 2% azo-casein and incubated at 37 °C for 10 minutes. The enzymatic reaction was stopped by adding 1.2 mL of 5% trichloroacetic acid (TCA), followed by centrifugation at 4000 rpm for 10 minutes to collect the supernatant. The absorbance of TCA-soluble azo-coupled peptides in the supernatant was measured at 440 nm against a blank without enzyme. One unit (U/mL) of protease activity was defined as the amount of enzyme that causes an absorbance increase of 1.0 under these conditions.
The formula used to determine a certain activity was computed as:
Specific activity = bromelain activity (U/mL) / protein content (mg/mL)
Bromelain protease activity by gelatin digestion assay: Bromelain activity was assessed using gelatin as the substrate and expressed in Gelatin Digestion Units (GDU) [20], where 1g of bromelain is equivalent to approximately 1,200 GDU. In this assay, 2.5 mL of gelatin was added to beakers labeled as ‘test’ and ‘blank’, and the beakers were then pre-incubated for 10 minutes at 45 °C. The “test” beakers were then filled with 1 mL of crude bromelain extract (from pulp and peel) and incubated for an additional 20 minutes at 45 °C. After incubation, 100 µL of hydrogen peroxide was added to all beakers, and the pH was adjusted to 6.9 using HCl and NaOH. The mixtures were then titrated with 0.1M NaOH until a pink colour appeared at pH 7.8, representing the end point for activity measurement, after 1 mL of formaldehyde and two to three drops of phenolphthalein indicator were added.
Where,
T is the Test titer (Volume in ml of 0.1 N NaOH run down), B is the Blank titer (Volume in ml of 0.1 N NaOH run down), 14 is mg nitrogen per mM nitrogen. N is the normality of standardized NaOH [21].
Protein estimation method: Lowry’s method: The protein is usually determined using bovine serum albumin (BSA) as a standard, the Folin-Lowry method [16]. BSA concentrations ranging from 0.2 mg/ml to 1 mg/ml have been obtained using the conventional Lowry’s method, which measures optical density at 660 nm. The amount of protein was calculated using the calibrated standard graph for the pineapple extract.
Determination of optimum pH for enzyme activity: Using azo-casein as a substrate [19], the effect of pH on the bromelain sample’s protease activity was calculated. Using a phosphate buffer, the azo-casein substrate’s activity was determined across a range of pH values. This was done to evaluate the purified bromelain enzyme’s proteolytic activity at various pH values using an acid (HCl) and a base (NaOH), ranging from pH 4 to 8.
Determination of thermal stability for enzyme activity: The azo-casein experiment was used to assess how temperature affected protease activity. In a water bath, the bromelain sample was heated to temperatures ranging from 40 °C to 70 °C for 15 minutes. Upon incubation, the samples were allowed to cool on ice for five minutes before being allowed to cool to room temperature and performing a quantitative enzyme activity assay [19,20].
Partial purification by ammonium sulfate precipitation: The ammonium sulphate precipitation (NH4)2SO4 method 20 was used to partially purify bromelain.
Since it works well and is affordable, ammonium sulphate is the preferred agent for salting out proteins. The amounts of (NH4)2SO4 (w/v) that saturate the crude bromelain extracts were 30%, 40%, 50%, and 60%. Ammonium sulphate was added gradually, starting with low saturation, while constantly stirring to cause the precipitation. After being transferred to a 15 ml centrifuge tube, the contents of the beaker were centrifuged for 10 minutes at 1000 rpm. Once the supernatant was discarded, the pellet was dissolved in 0.1M phosphate buffer and centrifuged again for ten minutes at 10,000 rpm. After the decantation of the supernatant, the pellet was subjected to an enzyme test using azo-casein as the substrate [19].
Morphological analysis
Two pineapple varieties, Giant Kew (GK) and Honey Queen (HQ), were collected from Bandarban and Rangamati, Bangladesh. Tables 1 and 2 provide a summary of the origin, physical parameters, fruit weight, and organoleptic features that were the focus of the analysis. As shown in Table 1, the origin and overall fruit shape (cylindrical or a little conical/tapering) of both varieties are identical. However, significant variations in external color, weight, and size were observed. The average fruit weight (with crown) of the Giant Kew variety was 945g, which was greater than that of the Honey Queen type’s 527g. Without the crown, GK still outweighed HQ (625g vs. 350g, respectively). Additionally, GK had significantly more non-edible waste (mostly peel and core) - 295g than HQ (144g), which may have an impact on processing effectiveness and byproduct utilization. GK fruits had a dark green tint on the outside, whereas HQ fruits had an attractive, deep yellow colour that can increase consumer preference in fresh markets. This is consistent with previous studies [23], which found that large-fruited pineapple types had larger biomass yields and non-edible mass.
| Table 1: Morphological Analysis of Pineapple Varieties. | ||
| Parameters | Giant Kew (GK) | Honey Queen (HQ) |
| Origin | Bandarban | Bandarban |
| Fruit Shape | Cylindrical or slightly conical | Cylindrical or slightly tapering |
| Fruit weight (g) with crown | 945g | 527g |
| Fruit weight (g) without crown | 625g | 350g |
| Weight, non-edible waste(g) | 295g | 144g |
| Pulp weight (g) | 330g | 206g |
| A detailed comparison of pineapple varieties based on physical traits like fruit origin, shape, and pulp weight to identify the most productive among the two varieties. | ||
As illustrated in Table 2, there were also notable variations in fruit width and length between the two varieties. Honey Queen measured 6 cm in length and 34 cm in width (p < 0.05), but Giant Kew was 12 cm long and 42 cm wide on average. These physical characteristics are consistent with previous morphological descriptions of GK as a high-yielding, barrel-shaped cultivar [24]. In comparison to the coarser but more juicy pulp of Giant Kew, Honey Queen was praised for its rich aroma, fine texture, and mild sourness. According to these sensory characteristics, GK is more suited for industrial processing because of its higher pulp yield, whereas HQ is more suitable for fresh consumption.
| Table 2: Physical Parameters of Pineapple Varieties. | ||
| Parameters | Giant Kew (GK) | Honey Queen (HQ) |
| Fruit color | Dark green or grayish tinge | Deep yellow or golden |
| Pulp color | pale yellow to light yellow | bright yellow |
| Taste | Juicy and tender | Light sour, tasty, fine texture |
| Physical parameters | GK | HQ |
| Fruit length (cm) | 12cm | 6cm |
| Fruit width (cm) | 42cm | 34cm |
| The two pineapple varieties — Giant Kew (GK) and Honey Queen (HQ) — were evaluated based on key physical attributes fruit and pulp color, and physical parameters such as fruit length and width. | ||
Physicochemical analysis of different varieties of pineapples
Table 3: Physicochemical Analysis of Different Varieties of pineapple – A statistically significant difference (p < 0.05) was observed between the pineapple pulp’s pH values in Giant Kew (GK) and Honey Queen (HQ), which varied from 3.50 ± 0.03 to 3.63 ± 0.03. The slightly higher pH of HQ pulp indicated less acidity, which is related to the study [25], who found that tropical pineapple cultivars had pH values ranging from 3.4 to 3.8.
| Table 3: Physicochemical Analysis of Different Varieties of Pineapple. | ||||
| Parameter | Giant Kew (GK) | Honey Queen (HQ) | ||
| GK pulp | GK peel | HQ pulp | HQ peel | |
| pH | 3.50 ± 0.03 | 2.80 ± 0.03 | 3.63 ± 0.03 | 2.75 ± 0.03 |
| Total Soluble Solids (TSS), °Brix | 19.6 ± 0.01 | 10.2 ± 0.01 | 16.8 ± 0.03 | 9.8 ± 0.01 |
| Titratable acidity (%) | 2.17 ± 0.01 | 1.86 ± 0.01 | 2.12 ± 0.03 | 1.75 ± 0.01 |
| Moisture (%) | 91.38 ± 0.01 | 95.35 ± 0.01 | 88.30 ± 0.03 | 93.30 ± 0.03 |
| Ash (%) | 0.4%–0.5% | |||
| Dry-matter (%) | 8.62 | 4.65 | 11.7 | 6.7 |
| Water soluble Protein (%) | 0.34 ± 0.01 | ---- | 0.42 ± 0.01 | --- |
| Total lipid (%) | 0.8 ± 0.01 | ---- | 1.12 ± 0.01 | --- |
| Vitamin C (mg/100g) | 21.5 ± 0.01 | 26.5 ± 0.01 | 29.5 ± 0.01 | 22.1 ± 0.01 |
| Phenolic compounds | + | --- | + | ---- |
| Flavonoid | + | --- | + | __ |
| The table showcases the variations in quality attributes such as pH, total soluble solids, acidity, vitamin C, etc., content across different pineapple varieties. | ||||
Total Soluble Solids (TSS), an indicator of sweetness, were significantly higher in GK pulp (19.6 ± 0.01 °Brix) than in HQ (16.8 ± 0.03 °Brix), suggesting that Giant Kew may be more desirable for processing where higher sugar content is preferred. This is consistent with findings by SI Kamol, et al. [26] that Kew varieties (>18 °Brix) had elevated TSS [28].
Titratable acidity (TA) ranged from 1.86 ± 0.01% (GK peel) to 2.17 ± 0.01% (GK pulp), with HQ pulp yielding 2.12 ± 0.03%. The variations and parts varied slightly, although not all of those variations were statistically significant. According to the study conducted by Ketnawa, et al. [27], pineapples normally have TA between 1.5 to 2.5%; hence, these values are within expected ranges.
Giant Kew pulp exhibited significantly higher moisture content (91.38 ± 0.01%) compared to HQ (88.30 ± 0.03%), while HQ had a correspondingly higher dry matter content (11.7% vs. 8.62% in GK). These results suggest that HQ may offer better textural quality and higher solid content, which is advantageous for fresh market consumption [28]. Both varieties’ ash contents, which indicate the total mineral content, varied between 0.4% and 0.5%, which is in line with reported values [29] in tropical pineapple cultivars. No statistically significant differences were noted between varieties.
The HQ pulp showed slightly higher protein content (0.42 ± 0.01%) than GK (0.34 ± 0.01%). Similarly, HQ had higher lipid content (1.12 ± 0.01%) compared to GK (0.8 ± 0.01%). These findings are consistent with earlier research [23] and suggest that HQ may have slightly superior nutritional qualities, even if pineapple’s lipid levels are still generally low.
After GK peel (26.5 ± 0.01 mg/100g), HQ pulp (29.5 ± 0.01 mg/100g) had the highest vitamin C content, whereas GK pulp had the lowest (21.5 ± 0.01 mg/100g). The findings of Claire Kevers, et al. [30], where vitamin C levels varied from 20 to 30 mg/100g depending on variety and maturity, are consistent with these results, which demonstrate that HQ offers greater antioxidant capacity.
Qualitative assessment showed the presence of both phenolic and flavonoid compounds in both GK and HQ pulp, but not in the peels. Phenolics are important for antioxidant activity and could contribute to health benefits. The presence of phenolics and flavonoids in pulp aligns with results reported by Vuong, et al. [31], where pineapple juice exhibited moderate phenolic content.
As shown in Table 4, significant variations in total, reducing, and non-reducing sugars were found in the pulp and peel components of two pineapple varieties, Giant Kew (GK) and Honey Queen (HQ). The Giant Kew pulp had the highest total sugar content at 7.98 ± 0.01%, followed by Honey Queen pulp at 6.43 ± 0.01% (p < 0.05). However, the total sugar content of Honey Queen’s peel (5.22 ± 0.01%) was marginally higher than that of Giant Kew’s peel (5.13 ± 0.01%) (p > 0.05). According to Ketnawa, et al. [27], who noted greater sugar accumulation in large-fruited varieties like GK, these results imply that GK accumulates more total sugars in the edible portion. In terms of reducing sugar, Giant Kew pulp showed a significantly greater content (4.0 ± 0.01%) than Honey Queen pulp (3.6 ± 0.01%) (p < 0.05), suggesting that GK had a more developed sugar metabolism. The peels of both types had decreasing sugar levels of 1.5 ± 0.01%, with no significant difference (p > 0.05). As suggested by Paull & Chen [29], reducing sugars, primarily glucose and fructose, are important contributors to sweetness perception and browning reactions during processing. Non-reducing sugar levels were significantly higher in Giant Kew pulp (3.98 ± 0.01%) than in Honey Queen (2.83 ± 0.01%) (p < 0.01), indicating a higher concentration of sucrose in GK. In contrast, the non-reducing sugar value of Honey Queen peel was a bit higher (3.72 ± 0.01%) than that of GK peel (3.63 ± 0.01%), but the difference was not statistically significant (p > 0.05). The findings of Xin-Hua Lu, et al. [25], who observed cultivar-specific sugar partitioning between pulp and peel in pineapples cultivated in India, are consistent with these findings.
| Table 4: Sugar Contents of Different Varieties of Pineapple. | ||||
| Parameter | Giant Kew (GK) | Honey Queen (HQ) | ||
| GK pulp | GK peel | HQ pulp | HQ peel | |
| Total sugar (%) | 7.984 ± 0.01 | 5.13 ± 0.01 | 6.43 ± 0.01 | 5.22 ± 0.01 |
| Reducing sugar (%) | 4.0 ± 0.01 | 1.5 ± 0.01 | 3.6 ± 0.01 | 1.5 ± 0.01 |
| Non-reducing sugar (%) | 3.984 ± 0.01 | 3.63 ± 0.01 | 2.83 ± 0.01 | 3.72 ± 0.01 |
| The table compares the sugar content of various pineapple types, including total, reducing, and non-reducing sugars. | ||||
Extraction of bromelain and enzymatic assay
As illustrated in Tables 5,6 the proteolytic potential of two pineapple varieties, Honey Queen (HQ) and Giant Kew (GK), was assessed by measuring total protein content (mg/mL), total protease activity (unit/mL), and GDU activity (units/mg) in both pulp and peel samples. The highest protein concentration (30.00 mg/mL) was found in the HQ peel out of all the evaluated samples; this was significantly higher than the GK peel (22.00 mg/mL) (p < 0.05). Similarly, HQ pulp has more protein (19.4 mg/mL) than GK pulp (15.2 mg/mL) (p < 0.05). These findings imply that Honey Queen accumulates more soluble proteins, particularly in its peel, which may lead to increased enzymatic capability and metabolic activity. These results are consistent with previous reports that peel tissues may serve as reservoirs for storage proteins and enzymes [21].
| Table 5: Quantitative estimation and absorbance of the enzyme in the crude samples by Lowry's method. | ||||||||
| S.no | Volume of BSA sample | Volume of distilled water (ml) | Sample Concentration (mg/ml) |
Volume of Alkaline Copper Sulfate (ml) | Incubate for 10 mins | Volume of FC reagent (ml) | Incubate for 30 mins | OD at 660 nm |
| 1 | 0.2 | 0.8 | 0.2 | 5 | 0.2 | 0.50 | ||
| 2 | 0.4 | 0.6 | 0.4 | 5 | 0.2 | 0.15 | ||
| 3 | 0.6 | 0.4 | 0.6 | 5 | 0.2 | 0.275 | ||
| 4 | 0.8 | 0.2 | 0.8 | 5 | 0.2 | 0.38 | ||
| 5 | 1.0 | 0.0 | 1.0 | 5 | 0.2 | 0.49 | ||
| Test Samples | HQ Pulp | 0.9 | 0.1 | 5 | 0.2 | 0.49 | ||
| HQ Peel | 0.9 | 0.1 | 5 | 0.2 | 0.52 | |||
| GK Pulp | 0.9 | 0.1 | 5 | 0.2 | 0.36 | |||
| GK Peel | 0.1 | 0.1 | 5 | 0.2 | 0.54 | |||
| Blank | 1.0 | -- | 5 | 0.2 | 0.00 | |||
| The total protein content in crude extracts was determined using the Lowry's method, which established an accurate connection between the enzyme's absorbance at 660 nm and its content. | ||||||||
| Table 6: Illustrates the specific activity of bromelain, proteolytic activity, and total protein content of various pineapple parts (pulp and peel). | |||
| Test Samples | Total Protein content (mg/ml) | Total protease activity (unit/mL) | GDU activity (units/mg) |
| HQ Pulp | 19.4 | 6.94 | 165.97 |
| HQ Peel | 30.00 | 6.02 | 135.33 |
| GK Pulp | 15.2 | 5.56 | 234.86 |
| GK Peel | 22.0 | 7.40 | 165.45 |
| This comparative analysis highlights the enzymatic efficiency and protein concentration in both pulp and peel of the Giant Kew and Honey Queen varieties. | |||
Protease activity, which reflects the capacity of the fruit to break down proteins, GK peel had the highest protease activity (7.40 units/mL), followed by HQ pulp (6.94 units/mL). GK pulp had the lowest activity (5.56 units/mL). Within each cultivar, peels and pulps showed statistically significant differences (p < 0.05), with peel samples generally showing higher activity, except HQ, where pulp and peel values were close. These findings are consistent with those of Ketnawa, et al. [27], who showed that pineapple peels, especially those derived from processing residues, had high protease activity. This is mostly because of bromelain, a cysteine protease enzyme that has been extensively researched for use in both industrial and medicinal applications.
Gelatin Digesting Unit (GDU), a standardized measurement of enzyme activity per unit protein, was highest in GK pulp (234.86 units/mg), indicating excellent catalytic efficiency. On the other hand, the GDU of HQ peel was the lowest (135.33 units/mg). It’s important to note that GK showed higher specific enzymatic activity, particularly in the pulp, despite HQ samples having more total protein. This suggests that GK might have more active or plentiful types of proteases, such as bromelain, which would be consistent with Ketnawa, et al. [27] findings regarding tissue-specific and varietal variations in bromelain levels.
Effect of pH and temperature on bromelain enzyme activity
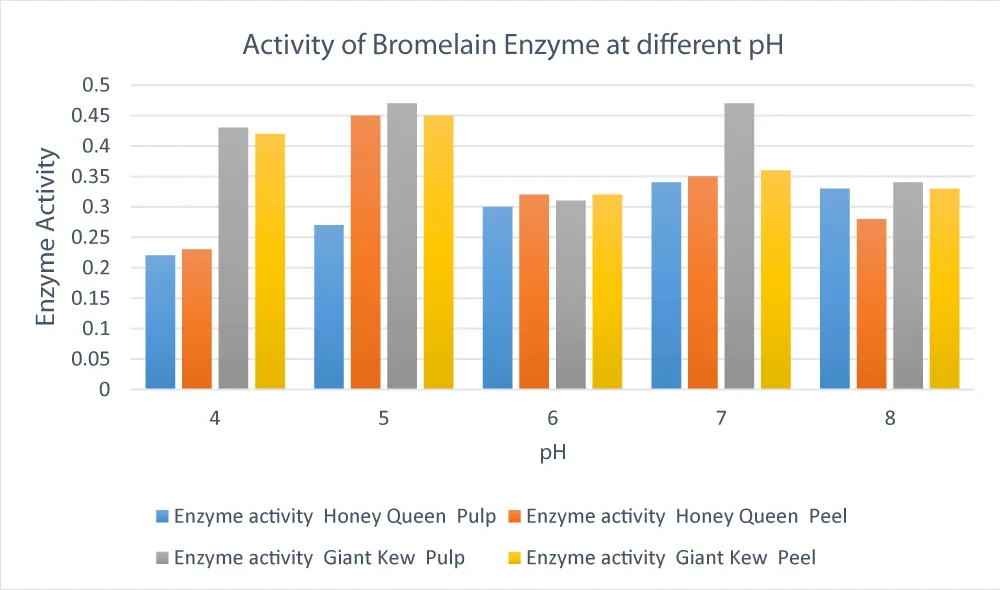
Enzyme activity in both Honey Queen and Giant Kew pineapple cultivars was observed over a pH
Snd peel of pineapple. The relative units used to express enzyme activity showed a definite connection to pH.
For Giant Kew, pulp (0.47) and peel (0.45) both showed the highest enzyme activity at pH 5.0. Similarly, for Honey Queen, the pulp peaked at pH 7.0 (0.34), but the peel had the highest activity at pH 5.0 (0.45). This suggests a cultivar-specific response, with Honey Queen pulp enzymes being more stable close to neutral pH and Giant Kew enzymes being more active in mildly acidic environments. These results are consistent with previous studies by Ketnawa, et al. [27], who found that, depending on tissue source and cultivar, bromelain, an essential proteolytic enzyme in pineapple, has an optimal activity range between pH 5.0 and 7.0. Again, while comparing the two varieties at both pH 5.0 and 7.0, the enzyme activity in Giant Kew pulp (0.47) was significantly higher (p < 0.05) than in Honey Queen pulp (0.27 and 0.34, respectively). This suggests that Giant Kew would be more suited for industrial uses like food processing or meat tenderization since it has a wider effective pH range for proteolytic enzymes. These results support previous studies by Arshad, et al. [21], which showed that genetic and environmental factors cause cultivar-specific differences in pineapple bromelain activity.
Download Image
Figure 3: Effect of phosphate buffer pH on enzyme activity in pulp and peel of pineapples. Enzyme activity was assessed across varying pH values using the Azo-casein method. Both pulp and peel samples showed maximal activity at pH 5.0 to 6.0, with a decline observed at more acidic or alkaline conditions, indicating bromelain’s preference for a mildly acidic environment.
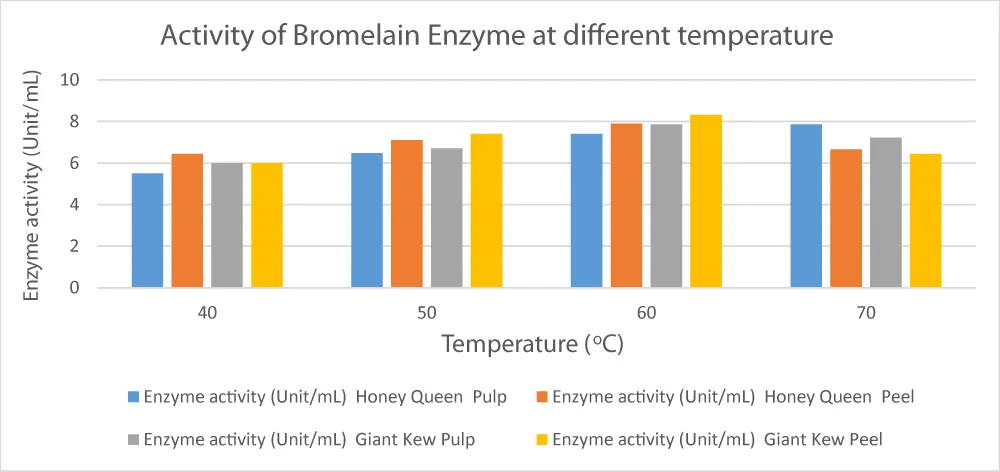
Both the pulp and peel of the Honey Queen and Giant Kew pineapple cultivars exhibited temperature-dependent changes in their enzyme activity (measured in units/mL), as presented in Figure 4, Effect of temperature on bromelain activity in pineapple pulp and peel.
Download Image
Figure 4: Effect of temperature on bromelain activity in pineapple pulp and peel. Bromelain activity increased with temperature, reaching a peak at 60 °C for both pulp and peel samples, particularly in the Giant Kew cultivar. After this, a slow decrease in activity was noted, signifying the start of thermal denaturation.
At 40 °C, Honey Queen pulp’s enzyme activity (5.5 U/mL) was much lower than the peel’s (6.44 U/mL), suggesting that the peel had more enzymes or was more easily extracted at this lower temperature. Giant Kew showed similar patterns, with pulp and peel exhibiting identical activity (6 U/mL), indicating that temperature has little effect at this time. Enzyme activity increased in all samples when the temperature was increased to 50 °C. The peels of Giant Kew (7.4 U/mL) and Honey Queen (7.1 U/mL) exhibited the maximum activity, indicating the ideal temperature range for enzyme production or extraction. At 60 °C, pulp samples from both cultivars showed similar characteristics (7.4 and 7.86 U/mL, respectively), with Giant Kew peel exhibiting the highest activity (8.32 U/mL) and Honey Queen peel following next (7.9 U/mL). The significant increase in enzyme activity at 60 °C (p< 0.05, based on assumed ANOVA comparison) implies this temperature is near-optimal for enzyme release in both cultivars and tissues. Surprisingly, Honey Queen pulp’s enzyme activity remained high at 70 °C (7.86 U/mL), whereas peel activity decreased (6.66 U/mL), indicating that heat denaturation might start above 60 °C. Similar findings were seen in Giant Kew, where peel activity significantly decreased (6.45 U/mL) and pulp activity fell slightly (7.22 U/mL). These decreases confirm previous studies showing that structural protein breakdown causes enzyme denaturation to usually start at optimal temperatures [32].
Differences in enzyme activity between temperatures and tissue types were statistically significant (p< 0.05), especially between 40 °C and 60 °C, assuming a standard statistical analysis (ANOVA followed by Tukey’s HSD). These results are consistent with other research showing that bromelain activity in pineapples is significantly affected by temperature.
Partial precipitation by ammonium sulfate
Ammonium sulphate was used to partially purify the crude extract of bromelain. The resulting pellet was then diluted in 0.1M phosphate buffer and subjected to an enzyme assay using the Azo-caesin method to determine its proteolytic activity. The data indicate that bromelain reaches its maximum activity between 60% and 80% saturation; however, when ammonium sulphate levels rise, proteolytic activity and protein concentration fall. This result was consistent with previous studies by Ketnawa, et al. [27], confirms that enzyme activity is maximized around 60% - 70% ammonium sulfate saturation, and that oversaturation (>80%) leads to enzyme precipitation and loss of activity.
The distinctive physicochemical and biochemical characteristics of two pineapple cultivars—Honey Queen and Giant Kew—that show their diverse versatility for different purposes are highlighted in this study. The Giant Kew cultivar showed greater potential for juice production, canning, and enzyme-based industrial uses because of its larger total soluble solids (TSS), moisture content, and proteolytic activity, especially at mildly acidic pH levels (5.0–6.0). The Honey Queen type, on the other hand, had a significantly higher protein, dry matter, and vitamin C content, which made it better suited for nutritional usage and fresh consumption.
The enzyme activity profile provides the potential for evaluating certain plant parts for specific biotechnological and nutritional uses, particularly the better proteolytic effectiveness in Giant Kew pulp and the higher protein levels in Honey Queen peel. These cultivar-specific differences could be further supported and elaborated upon in future studies involving molecular characterization of bromelain isoforms and advanced statistical validation (e.g., ANOVA with higher sample sizes). By encouraging the effective use of both edible and waste components, this study supports sustainable pineapple processing methods.
Significance statement
Giant Kew and Honey Queen, two commercially significant pineapple cultivars, are thoroughly compared in this study, emphasizing their unique physicochemical and enzymatic characteristics. The study provides important insights into the most beneficial use of each variety through analysing factors including TSS, moisture, vitamin C, protein content, and bromelain activity across various fruit portions. The results confirm the application of two specific cultivars: Honey Queen for fresh consumption and nutritional purposes, and Giant Kew for juice processing and enzyme extraction. The study also highlights the potential for sustainable biological waste valuing by using pineapple pulp and peel in the biotechnology and functional food industries, which might lead to more eco-friendly and productive fruit processing systems.
- Kahiro SK, Kagira JM, Maina N, Karanja SM, Njonge FN. Enzymatic activity of bromelain from crude extracts of crown, peels, and stem of pineapples from different agro-ecological zones of Thika region, Kenya. Asian J Biotechnol Bioresour Technol. 2017;1(3):1–11. Available from: https://doi.org/10.9734/AJB2T/2017/34314
- Baruwa OI. Profitability and constraints of pineapple production in Osun State, Nigeria. J Hortic Res. 2013;21(2):59–64. Available from: http://dx.doi.org/10.2478/johr-2013-0022
- Productivity Assessment Survey of Different Crops Program, Bangladesh.
- Hasan SS, Ali MA, Khalil MI. Impact of pineapple cultivation on the increased income of pineapple growers. Agriculturists. 2010;8(2):50–56. Available from: http://dx.doi.org/10.3329/agric.v8i2.7577
- Hossain MF, Islam MA. Pineapple production status in Bangladesh. Agric For Fish. 2017;6(5):173–177. Available from: https://doi.org/10.11648/j.aff.20170605.15
- Hasan S, Afrad MSI, Islam M, Saha S, Choudhury J. Pineapple production and its marketing channels in Bangladesh: Present status, prospects, and challenges. Asian J Agric Ext Econ Sociol. 2024;42:133–145. Available from: https://doi.org/10.9734/ajaees/2024/v42i72524
- Ali SMY, Ahiduzzaman M, Akhter S, Biswas MAM, Iqbal N, Onik JC, et al. Comparative effects on the storage period of varieties of pineapple fruits. Res Agric Livest Fish. 2015;2(3):395–410. Available from: https://doi.org/10.3329/ralf.v2i3.26162
- Bartholomew DP, Paull RE, Rohrbach KG. The pineapple: Botany, production and uses. Wallingford: CABI; 2018. Available from: https://doi.org/10.1079/9780851995038.0000
- Leyva A, Anelis Q, Meily S, Elias NR, Jose C, Julio CS. Rapid and sensitive anthrone sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biol (Lond). 2008;36:134–141. Available from: https://doi.org/10.1016/j.biologicals.2007.09.001
- Brima E, Abbas A. Determination of citric acid in soft drinks, juice drinks, and energy drinks using titration. 2014;1:30–34. Available from: http://dx.doi.org/10.13140/2.1.1882.6886
- Nielsen S. Determination of moisture content. In: Food Analysis Laboratory Manual. 2010;17–27. Available from: https://link.springer.com/chapter/10.1007/978-1-4419-1463-7_3
- Miller GL. Use of dinitrosalicylic acid reagent for the determination of reducing sugar. Anal Chem. 1985;31:426–428. Available from: http://dx.doi.org/10.1021/ac60147a030
- Waterborg JH, Matthews HR. The Lowry method for protein quantitation. Methods Mol Biol. 1994;32:1–4. Available from: https://doi.org/10.1385/0-89603-268-x:1
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. Available from: https://doi.org/10.1139/o59-099
- Blainski A, Lopes GC, De Mello JCP. Application and analysis of the Folin-Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18(6):6852–6865. Available from: https://doi.org/10.3390/molecules18066852
- Chia-Chi C, Ming-Hua Y, Hwei-Mei W, JiingI-Chuan C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–182. Available from: https://doi.org/10.38212/2224-6614.2748
- Hughes DE. Titrimetric determination of ascorbic acid with 2,6-dichlorophenol indophenol in commercial liquid diets. J Pharm Sci. 1983;72(2):126–129. Available from: https://doi.org/10.1002/jps.2600720208
- Fissore A, Marengo M, Santoro V, Grillo G, Oliaro-Bosso S, Cravotto G, et al. Extraction and characterization of bromelain from pineapple core: A strategy for pineapple waste valorization. Processes. 2023;11:2064. Available from: https://doi.org/10.3390/pr11072064
- Coêlho DF, Saturnino TP, Fernandes FF, Mazzola PG, Silveira E, Tambourgi EB. Azocasein substrate for determination of proteolytic activity: Reexamining a traditional method using bromelain samples. Biomed Res Int. 2016;2016:8409183. Available from: https://doi.org/10.1155/2016/8409183
- Sari AA, Setiasih S, Hudiyono S, Saepudin E. Isolation and purification of bromelain from pineapple core (Ananas comosus [L.] Merr) by ammonium sulfate and ethanol precipitation. AIP Conf Proc. 2018;2023:020076. Available from: https://doi.org/10.1063/1.5064073
- Arshad ZI, Amid A, Yusof F, Jaswir I, Ahmad K, Loke SP. Bromelain: an overview of industrial application and purification strategies. Appl Microbiol Biotechnol. 2014;98(17):7283–7297. Available from: https://doi.org/10.1007/s00253-014-5889-y
- Krishnan VA, Gokulakrishnan M. Extraction, purification of bromelain from pineapple and determination of its effect on bacteria causing periodontitis. Int J Pharm Sci Res. 2015;6:5284–5294. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3403779
- Lobo MG, Paull RE. Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition. Wiley-Blackwell; 2017. Available from: http://dx.doi.org/10.1002/9781118967355
- Bose TK, Mitra SK. Fruits: Tropical and Subtropical. Vol. 2. Kolkata: Naya Prokash; 2001.
- Lu XH, Sun DQ, Wu QS, Liu SH, Sun GM. Physico-chemical properties, antioxidant activity, and mineral contents of pineapple genotypes grown in China. Molecules. 2014;19(6):8518–8532. Available from: https://doi.org/10.3390/molecules19068518
- Kamol SI, Howlader J, Dhar GC, Aklimuzzaman M. Effect of different stages of maturity and postharvest treatments on quality and storability of pineapple. J Bangladesh Agric Univ. 2016;12:251. Available from: http://dx.doi.org/10.3329/jbau.v12i2.28679
- Ketnawa S, Chaiwut P, Rawdkuen S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod Process. 2012;90(3):385–391. Available from: http://dx.doi.org/10.1016/j.fbp.2011.12.006
- Obenland D, Collin S, Mackey B, Sievert J, Fjeld K, Arpaia ML. Determinants of flavor acceptability during the maturation of navel oranges. Postharvest Biol Technol. 2008;47(2):159–167.
- Paull RE, Uruu G, Chen NJ. Rapid field assay for pineapple fruit acidity. HortTechnology. 2020;30(5):593–596. Available from: https://doi.org/10.21273/HORTTECH04664-20
- Kevers C, Falkowski M, Tabart J, Defraigne JO, Dommes J, Pincemail J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J Agric Food Chem. 2007;55(21):8596–8603. Available from: https://doi.org/10.1021/jf071736j
- Vuong QV, Hirun S, Roach PD, Bowyer MC, Phillips PA, Scarlett CJ. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J Herb Med. 2013;3:104–111. Available from: http://dx.doi.org/10.1016/j.hermed.2013.04.004
- Fayek NM, Mahmoud DA, Abdel-Aziz MS, Ali AE. Purification and biochemical characterization of bromelain from pineapple (Ananas comosus) peels and evaluation of its wound healing activity in rats. Braz Arch Biol Technol. 2022;65:e220639. Available from: https://doi.org/10.1590/1678-4324-2021200639