More Information
Submitted: October 23, 2025 | Approved: October 25, 2025 | Published: October 27, 2025
How to cite this article: Attri A, Arya V. Therapeutic Applicability of Fruticose Lichens: A Brief Review on Pseudevernia furfuracea. J Plant Sci Phytopathol. 2025; 9(3): 090-096. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001160
DOI: 10.29328/journal.jpsp.1001160
Copyright license: © 2025 Attri A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Lichen; Anti-cancer; Pseudevernia furfuracea; Tree moss; Embalming; Biomonitoring
Therapeutic Applicability of Fruticose Lichens: A Brief Review on Pseudevernia furfuracea
Anshul Attri1 and Vikrant Arya2*
and Vikrant Arya2*
1Department of Pharmaceutical Sciences, Government College of Pharmacy, Rohru, Shimla, Himachal Pradesh, 171207, India
2Department of Pharmaceutical Sciences, Government Pharmacy College Rakkar, District Kangra, Himachal Pradesh, 177043, India
*Address for Correspondence: Vikrant Arya, Department of Pharmaceutical Sciences, Government Pharmacy College Rakkar, District Kangra, Himachal Pradesh, 177043, India, Email: [email protected]
The distinctness in the chemical profile of fruticose lichens marked their versatile applications from culinary, dyeing, to modern therapeutics. Pseudevernia furfuracea, commonly known as Tree Moss, has been used from the Ancient Egyptian era to the Modern World. It has exemplary applicability in embalming the dead bodies and has also been used in perfume-making industries. It is a rich source of distinct bioactive constituents reported to exert different Pharmacological activities. Constituents, including depsides, depsidones, orsellinic acid derivatives, pulvinic acid derivatives, aromatic polyketides, and phenolic acids, have been identified in the species via chromatography and spectroscopic approaches. It has been reported to exhibit anticancer, antioxidant, antimicrobial, anti-inflammatory, enzyme-inhibiting, and antifungal activities. Apart from its reported bioactivities, its biomonitoring potential further strengthens the significance of this species.
The healing properties of nature remarkably affect humans in the path of their evolution [1]. Curative principles present in medicinal plants, lichens, and other life forms offer a plethora of compounds capable of exerting diverse bioactivities [2]. They are not only involved in a preventive role but also can modulate the bodily function at the molecular level, thereby providing a permanent solution to a disease and disorder [3]. The applications of lichens in therapeutics led to the discovery of some important lichen-derived constituents. Lichen bioactive constituents, viz. usnic acid, salazinic acid, atranorin, gyrophoric acids, lecanoric acid, and stictic acid, showed their potential in several diseases [4]. Some commonly therapeutically active lichens are Cetraria aculeata, Cladonia chlorophaea, Pseudevernia furfuracea, Rhizoplaca marginalis, and Xanthoria parietina [5]. Literature review revealed their potential as antioxidants [6], anticancer [7], antimicrobial [8], anti-inflammatory [9], anti-fungal [10], anti-diabetic [11], etc. Some lichens showed a protective role in immune-related disorders [12]. Varied lichens have also been used as nutraceuticals due to the presence of vitamins, minerals, amino acids, proteins, etc. [13]. Apart from their therapeutic applications, lichens have been utilized in the preservation of food [14], the dyeing of fabrics [15], perfumery [16], and for their inherent ecological roles [17]. Historical records of the Egyptian era have deciphered some of their extraordinary applications, such as the mummification of dead bodies [18]. Several lichens served as an essential ingredient used in the preservation of mummies [19]. The expertise of the Ancient Egyptians in preservation facilitated the use of lichens in preserving dead bodies, hence decoding their diverse applications in mummification [20]. Several liches are extremophilic and have been associated with terraforming Mars and might answer to the human quest to survive on outer planets [21]. The environmental applications of lichens in biomonitoring are indispensable. Their inherent biosensing ability promoted their usage in environmental biomonitoring [22].
A comprehensive literature search was conducted across scientific databases, viz. Google Scholar, PubMed, and Web of Science. This exploration utilized several keywords relevant to the topic, including ‘tree moss’, ‘fruticose lichens’, ‘Pseudevernia furfuracea’, and ‘Pharmacology of Pseudevernia furfuracea’, among others. This systematic approach ensured wide coverage of the selected topic and paved the way for further investigation.
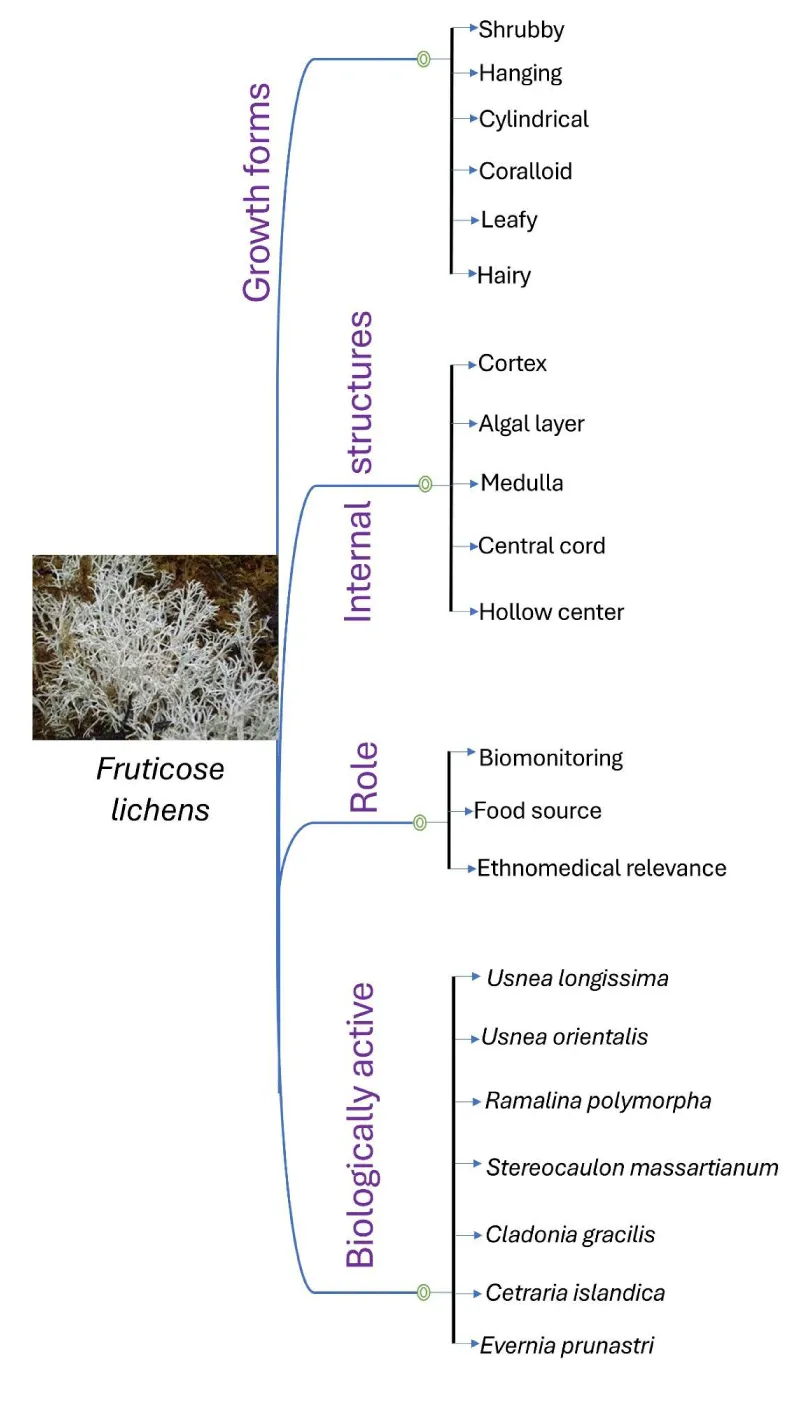
Fruticose lichens
Fruticose lichens evolve in such a way to cope with natural stressors, which is reflected in their existing morphological traits. The shrub-like, branched thallus possesses an outermost protective layer (cortex). With some exceptions, the photosynthetic algal layer is often present beneath the cortex, responsible for performing vital functions. The medullary layer (loosely packed fungal hyphae), involved in gaseous exchange and storage tissue for water and metabolites, forms the bulk of the thallus. Some fruticose lichens also possess a specialised central cord providing mechanical strength [23-25]. Fruticose lichens existed in varied life forms, viz., shrubby (Pseudevernia furfuracea), hanging (Alectoria sarmentosa), cylindrical (Cladonia), coralloid (Tuckermannopsis), hairy (Usnea spp.), etc. [26-28]. Some common examples of fruticose lichens include Dendriscocaulon intricatulum, Siphula ceratites, Polychidium muscicola, Aspicilia hispida, Caloplaca coralloides, Cornicularia normoerica, Hubbsia parishii, and Lecanora phryganitis [28]. They have a profound role in biomonitoring [29] and also serve as a food source for a variety of animals [30] and birds [31].
Fruticose lichens were also reported to exert a wide array of biological activities in relevance to their ethnomedicinal uses, as represented in Figure 1 & Table 1, viz., antioxidant (Evernia prunastri), anti-cancer (Usnea longissima), anti-inflammatory (Cladonia gracilis), anti-viral (Cetraria islandica), antimicrobial (Usnea undulata), and Antidermatophytic (Usnea orientalis) [32-41].
| Table 1: Common examples of therapeutically active fruticose lichens. | |||
| Common name | Fruticose lichen | Pharmacology | Reference |
| Oakmoss | Evernia prunastri, Parmeliaceae |
Antimicrobial, antioxidant | [32] |
| Iceland moss | Cetraria islandica, Parmeliaceae |
Antiviral | [33] |
| Old man's beard | Usnea baileyi, Parmeliaceae |
Anticancer | [34] |
| Beard lichen | Usnea orientalis, Parmeliaceae |
Antidermatophytic | [35] |
| Methuselah's beard lichen | Usnea longissima, Parmeliaceae |
Anticancer | [36] |
| Undulated old man's beard | Usnea undulata, Usneaaceae |
Antibacterial | [37] |
| Smooth cup lichen | Cladonia gracilis, Cladoniaceae |
Antioxidant, anti-inflammatory |
[38] |
| Snow lichen | Stereocaulon massartianum, Stereocaulaceae |
Antioxidant | [39] |
| Granular bush lichen | Ramalina polymorpha, Remalinaceae |
Antioxidant | [40] |
| - | Ramalina dendriscoides, Remalinaceae |
Antimicrobial | [41] |
Botanical description of Pseudevernia furfuracea
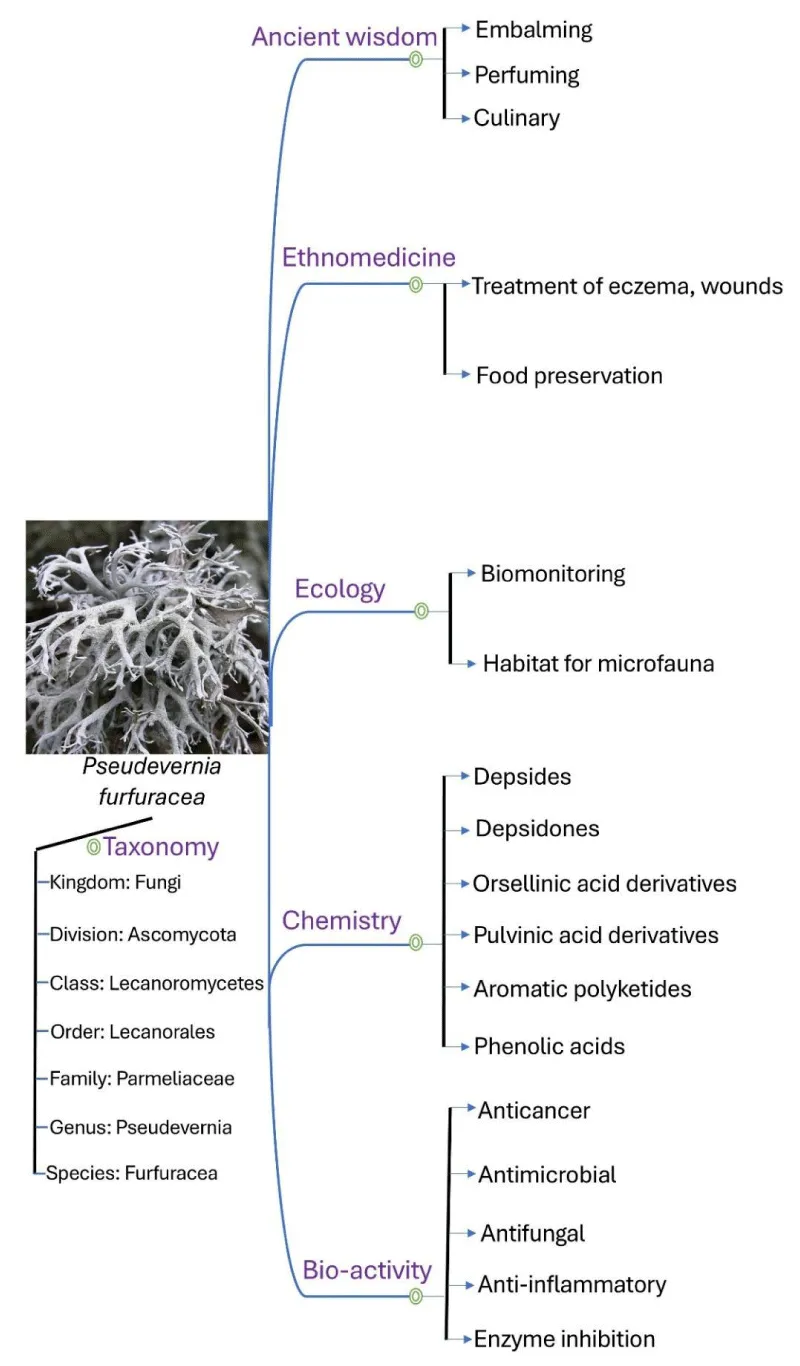
The tree moss (Pseudevernia furfuracea) exists in a symbiotic association among algae (Trebouxia) and fungus and serves as a unique habitat for microfauna. It dwells on the bark of trees like birch, pine, and spruce [42], and rarely on saxicolous form [43]. This fruticose lichenized species predominantly inhabits cool-temperate regions [44]. The thallus, having forked lobes, has a silver grey or light greyish upper surface and a black mottled lower surface. The peg-like isidia of P. furfuracea involved in vegetative reproduction emerge as the propagules of the lichen cortex [45,46].
Ethnomedicinal uses
Traditionally, it has been used in the treatment of wounds, eczema, and several conditions of respiratory, intestinal weakness. Literature revealed the usage of this foliose lichen by ethnic people in culinary practices and food preservation due to its aromatic properties [47].
Chemistry of P. furfuracea
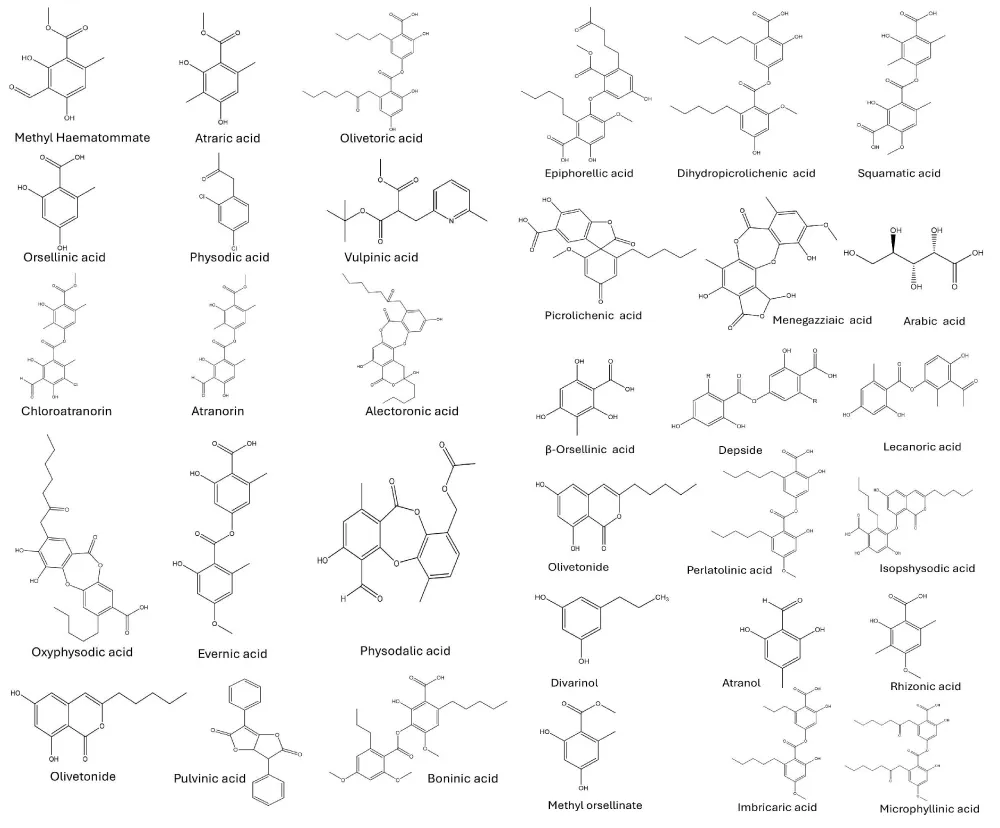
Pseudevernia furfuracea contains a reservoir of distinctive compounds capable of exhibiting a wide array of pharmacological activities, as represented in Figure 3. Lichen majorly contains depsides (squamatic acid, olivetoric acid, atranorin, chloroatranorin, boninic acid, lecanoric acid, dihydropicrolichenic acid, imbricaric acid, microphyllinic acid), depsidones (alectroronic acid, physodalic acid, oxyphysodic acid, menegazziaic acid), orsellinic acid derivatives (β-orsellinic acid, methyl orsellinate, orsellinic acid), phenolic acids (atraric acid, evernic acid), polysaccharides (Arabic acid), aromatic polyketides (olivetonide), hydroxybenzaldehydes (atranol), etc. [48-55].
Pharmacological status of P. furfuracea
The methanolic extract of tree moss (P. furfuracea) demonstrated its anti-metastatic potential in colorectal carcinoma (HCT-116, SW-480) by inhibiting the migration and invasion of respective cell lines [56]. Petrova, et al. [57] reported the effectiveness of acetone extract and physodic acid in a selected breast cancer cell line, i.e., MCF-10A. Atraric acid reported in P. furfuracea demonstrates significant activity against PA-1 cells (ovarian cancer), inhibiting their growth and potentially acting as an EGFR inhibitor [48]. Lichen-derived compounds (atraric acid, methyl hematommate, methyl chlorohematommate) and active fractions showed anti-inflammatory potential [50]. Mitrovik, et al. [49] reported antioxidative, antimicrobial, and antibiofilm potentials of various extracts of P. furfuracea and found proficient results. Several lichen-derived constituents (evernic acid, usnic acid, physodalic acid) showed potential when evaluated in vitro involving antioxidant and antibacterial activities [53]. Physodic acid isolated from tree moss showed its effectiveness in haematological malignancies, thereby inhibiting apoptosis in Jurkat cells [54]. The aqueous extract of P. furfuracea revealed its potential in oxidative damage when evaluated in vitro [58]. Essadki, et al. [59] detected the presence of several volatile constituents capable of exerting antimicrobial activities against multidrug-resistant bacteria. The ethanolic extract of P. furfuracea revealed its relevance as an antifungal candidate evaluated in vitro [60] along with enzyme inhibitory activities [52]. The phytopharmacological potential of tree moss has been represented in Table 2, Figure 2.
| Table 2: Pharmacological status of P. furfuracea. | |||
| Extract /Derived compound | Targeted study area | Outcome | Reference |
| Methanol extract | Colorectal carcinoma (HCT-116, SW-480) |
Inhibition of cancer cell migration | [56] |
| Acetone extract, Physodic acid | Breast cancer (MCF-10A) |
Suppression of TGF-β signalling | [57] |
| Atraric acid | Ovarian cancer (PA-1) |
Atraric acid-mediated inhibition of EGFR | [48] |
| Atraric acid, methyl hematommate, methyl chlorohematommate | Anti-inflammatory | Fractions containing active constituents displayed significant anti-inflammatory activities. | [50] |
| Extracts (acetone, ethyl acetate, methanolic extract) | Antioxidative, antimicrobial, antifungal | Extracts showed prominent activities viz. antioxidant (IC50 95.33 µg/mL), antimicrobial (MIC 0.005 mg/mL to 2.5 mg/mL), antifungal (0.04 mg/mL to 2.5 mg/mL) | [49] |
| Evernic acid, usnic acid, physodalic acid | Antibacterial, antioxidant | Extracts showed prominent activities as determined by their MIC values | [53] |
| Physodic acid | Anti-leukemic | Physodic acid induced apoptosis in Jurkat cells | [54] |
| Aqueous extract | Antioxidant | Significant improvement in the antioxidant defense system and prominent reduction in oxidative stress associated with type 1 diabetic rats | [58] |
| Volatile compounds | Antimicrobial | Volatile compounds showed activity against multidrug-resistant bacteria, fish pathogens, and Candida albicans | [59] |
| Ethanolic extract | Antifungal | The extract showed prominent antifungal potential against the selected Aspergillus strains. | [60] |
Other uses
Tree moss (Pseudevernia furfuracea) has been used in culinary and perfumery since ancient times to the present day [61]. Its characteristic woody odour and fixative properties aid its usage in perfumery [62]. Major phenolic volatile compounds have been identified in this species by GC-MS, contributing to its odorous properties [50].
The fruticose lichen P. furfuracea has been widely used to monitor trace elements and environmental contaminants in several locations in Italy, contributing to its biosensing properties. The major locations in Italy were reported as Torino, Bergamo, Sondrio, Trento, Bolzano, Belluno, Udine, Lucca, and Catanzaro [63], as well as the urban area in Naples [64]. The outer locations outside Italy where the biomonitoring potential of this lichen was established are Central Anatolia [65], Slovakia [66].
The ancient Egyptian practice of preserving dead bodies involved injecting solutions containing natural substances, such as resins, gums, oils, and lichens, as well as inorganic materials [67]. The historic text highlights the significance of P. furfuracea in the preservation of dead bodies [68,69], as it has been found stuffed inside the corpse’s body cavities and also detected in the linen cloth used for wrapping mummies [70].
Safety considerations
Rapid metallic pollution raised due to industrialization and increased man-made activities tends to alleviate the presence of toxic elements in the environment [71]. Lichens, being bioaccumulators, absorb pollutants [72], which can lead to toxicity in them [73]. It is necessary to conduct quantitative heavy metal detection from sophisticated instrumentation methods like Inductively Coupled Plasma Mass Spectrometry, Atomic Absorption Spectroscopy, before its inclusion in any product [74].
The current communication highlighted the potential of fruticose lichen P. furfuracea from an ecological to a therapeutic perspective. The ancient use of P. furfuracea in embalming the dead bodies and perfume making revealed its commercial importance in the Egyptian era. The biosensing properties of this species further mark its ecological importance in monitoring pollution. The species is found to be a rich source of distinctive depsides, phenolic acids capable of eliciting a wide array of pharmacological activities. The literature revealed its significance as an important antioxidant, anti-inflammatory, and anticancer candidate. Keeping in view its biomonitoring and therapeutic applications, the tree moss P. furfuracea should be further explored to harness its complete potential. However, the consideration of several factors, viz., heavy metal content, toxicity prediction, and accurate identification of this species should be monitored to ensure its safe usage in therapeutics.
Author’s contribution
Vikrant Arya (VA) initiated the conceptualization of the review article and is responsible for the overall research design, guiding the scope and direction of the work. Anshul Attri (AA) was primarily responsible for drafting the manuscript, ensuring comprehensive coverage of the literature. Both VA and AA collaboratively revised and approved the final version of the article, contributing equally to its intellectual content and accuracy.
We extend our sincere gratitude to eminent Professor Vivek Sharma, Director/Principal of Government College of Pharmacy Rohru, Himachal Pradesh, for his unwavering support and encouragement throughout this endeavour. Additionally, we would like to thank Heighten Science Publications Corporation (HSPI), USA, for their consideration of our article. Their platform provides valuable opportunities for sharing research and contributing to the scientific community.
- Ibrahim NI, Wong SK, Mohamed IN, Mohamed N, Chin KY, Ima-Nirwana S, et al. Wound healing properties of selected natural products. Int J Environ Res Public Health. 2018;15(11):2360. Available from: https://doi.org/10.3390/ijerph15112360
- Ahmed MZ, Rao T, Khan NA, Aslam M, Pane YS. Antimicrobial activities of lichens. In: Chemistry, Biology, and Pharmacology of Lichen. 2024;169-91. Available from: https://doi.org/10.1002/9781394190706.ch13
- Paguirigan JA, Liu R, Im SM, Hur JS, Kim W. Evaluation of antimicrobial properties of lichen substances against plant pathogens. Plant Pathol J. 2022;38(1):25. Available from: https://doi.org/10.5423/PPJ.OA.12.2021.0176
- Rankovic B, Kosanic M. Lichens as a potential source of bioactive secondary metabolites. In: Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential. Cham: Springer International Publishing; 2019;1-29. Available from: https://link.springer.com/chapter/10.1007/978-3-319-13374-4_1
- Karagoz Y, Karagoz BO. Lichens in pharmacological action: what happened in the last decade? Eurasian J Med. 2022;54(Suppl 1): S195. Available from: https://doi.org/10.5152/eurasianjmed.2022.22335
- Kosanic M, Rankovic B, Vukojevic J. Antioxidant properties of some lichen species. J Food Sci Technol. 2011;48(5):584-90. Available from: https://link.springer.com/article/10.1007/s13197-010-0174-2
- Solarova Z, Liskova A, Samec M, Kubatka P, Busselberg D, Solar P. Anticancer potential of lichens' secondary metabolites. Biomolecules. 2020;10(1):87. Available from: https://doi.org/10.3390/biom10010087
- Tian L, Wang T, Luan L, Meng Z, Han J, Zhao C, et al. Terminally Symmetric β-Turn Peptides for Multidrug-Resistant Bacterial Infections. J Med Chem. 2025;68(9):9341-56. Available from: https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.4c03057
- Nguyen HT, Ketha A, Kukavica B, Tatipamula V. Anti-inflammatory potential of lichens and their substances. MedDocs Ebooks. 2021;1-9. Available from: https://doi.org/10.1021/acs.jmedchem.4c03057
- Furmanek L, Czarnota P, Seaward MR. A review of the potential of lichen substances as antifungal agents: the effects of extracts and lichen secondary metabolites on Fusarium fungi. Arch Microbiol. 2022;204(8):523. Available from: https://doi.org/10.1007/s00203-022-03104-4
- Thadhani VM, Karunaratne V. Potential of lichen compounds as antidiabetic agents with antioxidative properties: a review. Oxid Med Cell Longev. 2017;2017(1):2079697. Available from: https://doi.org/10.1155/2017/2079697
- Shrestha G, St Clair LL, O'Neill KL. The immunostimulating role of lichen polysaccharides: a review. Phytother Res. 2015;29(3):317-22. Available from: https://doi.org/10.1002/ptr.5251
- Thakur M, Kasi IK, Islary P, Bhatti SK. Nutritional and health-promoting effects of lichens used in food applications. Curr Nutr Rep. 2023;12(4):555-66. Available from: https://doi.org/10.1007/s13668-023-00489-6
- David RLM, Thajuddin S, Moorthy IG, Dhanasekaran D, Kumar RS, Thajuddin N. Processed lichens could be a potential functional food with special reference to traditional dishes. In: Fermented Food Products. CRC Press. 2019;67-76. Available from: https://doi.org/10.1201/9780429274787-5?urlappend=%3Futm_source%3Dresearchgate
- Mendili M, Aschi-Smiti S, Khadhri A. Phytochemical screening of natural textile dyes extracted from Tunisian lichens. Biomass Convers Biorefin. 2025;15(1):1443-60. Available from: https://doi.org/10.1007/s13399-023-05135-3
- Das AK, Sharma A, Kathuria D, Ansari MJ, Bhardwaj G, editors. Chemistry, Biology, and Pharmacology of Lichen. Hoboken (NJ): John Wiley & Sons; 2024. Available from: https://www.wiley.com/en-us/Chemistry%2C+Biology+and+Pharmacology+of+Lichen-p-9781394190690
- Ellis CJ, Asplund J, Benesperi R, Branquinho C, Di Nuzzo L, Hurtado P, et al. Functional traits in lichen ecology: a review of challenge and opportunity. Microorganisms. 2021;9(4):766. Available from: https://www.mdpi.com/2076-2607/9/4/766
- Abdel-Maksoud G, El-Amin AR. A review of the materials used during the mummification processes in ancient Egypt. Mediterr Archaeol Archaeom. 2011;11(2):129-50. Available from: https://www.researchgate.net/publication/281404720_A_REVIEW_ON_THE_MATERIALS_USED_DURING_MUMMIFICATION_PROCESSES_IN_ANCIENT_EGYPT
- Bogdon E. Uses of plants in burial practices. In: Forensic Botany. CRC Press. 2024;103-11.
- Agai JM. The role of the ancient Egyptians' beliefs in the afterlife in preserving the ancient Egyptian cultural heritage. J Lang Cult. 2020;11(1):17-23. Available from: https://academicjournals.org/journal/JLC/article-full-text-pdf/B240B5D64139
- Armstrong RA. The lichen symbiosis: lichen “extremophiles” and survival on Mars. J Astrobiol Space Sci Rev. 2019;1:378-97. Available from: https://www.researchgate.net/publication/334603822_The_Lichen_Symbiosis_Lichen_Extremophiles_and_Survival_on_Mars
- Shukla V, Baipai R, Upreti DK. Lichens to Biomonitor the Environment. Springer India; 2014. Available from: https://link.springer.com/book/10.1007/978-81-322-1503-5
- Nash TH. Lichen Biology. 2nd ed. Cambridge University Press; 2008. Available from: https://api.pageplace.de/preview/DT0400.9780511410826_A23678173/preview-9780511410826_A23678173.pdf
- Gargas A, DePriest PT. Phyletic relationships among species of Usnea (lichenized Ascomycetes) inferred from ITS ribosomal DNA sequence data. Syst Bot. 1996;21(3):333-40.
- Notov AA. Fruticose lichens: structural diversity, taxonomic characteristics and evolution. Wulfenia. 2014;21(5):21-31. Available from: https://www.zobodat.at/pdf/Wulfenia_21_0021-0031.pdf
- Aptroot A, Barreto FMO, Pena DAR, da Silva Caceres ME. A new lineage of fruticose lichens that belongs to the Trapeliaceae (Trapeliales, Ascomycota) from Alagoas, NE Brazil. Bryologist. 2018;121(4):529-35. Available from: https://doi.org/10.1639/0007-2745-121.4.529
- Galun M, editor. CRC Handbook of Lichenology. Vol. 2. Boca Raton: CRC Press. 1988;153-69. Available from: https://dalspaceb.library.dal.ca/server/api/core/bitstreams/beb5fde6-571d-433b-9c5b-29f1f72f5b37/content
- Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. Yale University Press; 2001. Available from: https://www.researchgate.net/publication/341694944_Lichens_of_North_America
- Sueoka Y, Sakakibara M, Sano S, Yamamoto Y. A new method of environmental assessment and monitoring of Cu, Zn, As, and Pb pollution in surface soil using terricolous fruticose lichens. Environments. 2016;3(4):35. Available from: https://doi.org/10.3390/environments3040035
- Yang MX, Devkota S, Wang LS, Scheidegger C. Ethnolichenology-the use of lichens in the Himalayas and southwestern parts of China. Diversity. 2021;13(7):330. Available from: https://doi.org/10.3390/d13070330
- Nogales M, Hervias-Parejo S. Consumption of the lichen Roccella gracilis by the large ground-finch Geospiza magnirostris on the island of Daphne Major (Galapagos). Ornitol Neotrop. 2023;34(1):40-1. Available from: https://doi.org/10.58843/ornneo.v34i1.1161
- Shcherbakova A, Stromstedt AA, Goransson U, Gnezdilov O, Turanov A, Boldbaatar D, et al. Antimicrobial and antioxidant activity of Evernia prunastri extracts and their isolates. World J Microbiol Biotechnol. 2021;37(8):129. Available from: https://doi.org/10.1007/s11274-021-03099-y
- Makarevich EV, Filippova EI, Sedel’nikova NV, Mazurkov OY, Protsenko MA, Shishkina LN, et al. Anti-influenza activity of Cetraria islandica lichen extracts in in vitro experiments. Bull Exp Biol Med. 2023;175(2):215-8. Available from: https://link.springer.com/article/10.1007/s10517-023-05837-8
- Mariraj M, Gundappa M, Velayuthaprabhu S, Shah K, Ponnuchamy P, Mendem SK, et al. In vitro, in vivo, and in silico anticancer activity and toxicity of Usnic acid extracted from the mycobiont culture of Usnea baileyi. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(5):5101-17. Available from: https://link.springer.com/article/10.1007/s00210-024-03584-9
- Pathak A, Upreti DK, Dikshit A. Antidermatophytic activity of the fruticose lichen Usnea orientalis. Medicines. 2016;3(3):24. Available from: https://doi.org/10.3390/medicines3030024
- Engin TA, Emsen B, Yılmaz R, Koc RC, Inan B, Ozcimen D. Cytotoxicity of Usnea longissima Ach. Extracts and their secondary metabolite, usnic acid, on different cells. Anat J Bot. 2023;7(2):140-5. Available from: https://doi.org/10.30616/ajb.1343823
- Sultana N, Afolayan AJ. A new depsidone and antibacterial activities of compounds from Usnea undulata Stirton. J Asian Nat Prod Res. 2011;13(12):1158-64. Available from: https://doi.org/10.1080/10286020.2011.622720
- Torres-Benitez A, Ortega-Valencia JE, Sanchez M, Hillmann-Eggers M, Gomez-Serranillos MP, Vargas-Arana G, et al. UHPLC-MS chemical fingerprinting and antioxidant, enzyme inhibition, anti-inflammatory in silico and cytoprotective activities of Cladonia chlorophaea and C. gracilis (Cladoniaceae) from Antarctica. Antioxidants. 2022;12(1):10. Available from: https://doi.org/10.3390/antiox12010010
- Ismed F, Devehat FLL, Rouaud I, Ferron S, Bakhtiar A, Boustie J. NMR reassignment of stictic acid isolated from a Sumatran lichen Stereocaulon montagneanum (Stereocaulaceae) with superoxide anion scavenging activities. Z Naturforsch C. 2017;72(1-2):55-62. Available from: https://doi.org/10.1515/znc-2016-0148
- Türkez H, Aydın E, Aslan A. Effects of Lichenic Extracts (Hypogymnia physodes, Ramalina polymorpha and Usnea florida) on Human Blood Cells: Cytogenetic and Biochemical Study. Iran J Pharm Res. 2012;11(3):889-96. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3813136/
- Santiago K, Borricano J, Canal JN, Marcelo DMA, Pérez MCP, de la Cruz TEE. Antibacterial activities of fruticose lichens collected from selected sites in Luzon Island, Philippines. Philipp Sci Lett. 2010;3(2):18-29. Available from: https://www.semanticscholar.org/paper/Antibacterial-activities-of-fruticose-lichens-from-Angelique-Santiago/cc476c498d2634dba2d42c6f01d01663d6ac7629
- Robeck A. Pseudevernia Furfuracea-Patterns of Diversity in a Shrubby Lichen. Huddinge: Institutionen for livsvetenskaper; 2007. Available from: https://www.diva-portal.org/smash/get/diva2:15388/FULLTEXT01.pdf
- Aguirre-Hudson B, Whitworth I, Spooner BM. JM Despreaux's from the Canary Islands and West Africa: an account of a 19th-century collection found in an English archive. Bot J Linn Soc. 2011;166(2):185-211. Available from: https://doi.org/10.1111/j.1095-8339.2011.01140.x
- Malaspina P, Modenesi P, Giordani P. Physiological response of two varieties of the lichen Pseudevernia furfuracea to atmospheric pollution. Ecol Indic. 2018;86:27-34. Available from: https://doi.org/10.1016/j.ecolind.2017.12.028
- Rikkinen J. Habitat shifts and morphological variation of Pseudevernia furfuracea along a topographic gradient. Symb Bot Upsal. 1997;1997(32):223-45. Available from: https://www.researchgate.net/publication/235637167_Habitat_shifts_and_morphological_variation_of_Pseudevernia_furfuracea_along_a_topographic_gradient
- Tretiach M, Crisafulli P, Pittao E, Rinino S, Roccotiello E, Modenesi P. Isidia ontogeny and its effect on the CO2 gas exchanges of the epiphytic lichen Pseudevernia furfuracea (L.) Zopf. Lichenologist. 2005;37(5):445-62. Available from: https://doi.org/10.1017/S0024282905014982
- Ahmad, S., Katiyar, C. K., Ulrich-Merzenich, G. S., & Mukherjee, P. K. (2022). Metabolomics and ethnopharmacology in the development of herbal and traditional medicine. Frontiers in Pharmacology, 13, 851023. Available from: https://doi.org/10.3389/fphar.2022.851023
- Kalra R, Conlan XA, Areche C, Dilawari R, Goel M. Metabolite profiling of the Indian food spice lichen, Pseudevernia furfuracea, combined with optimised extraction methodology to obtain bioactive phenolic compounds. Front Pharmacol. 2021;12:629695. Available from: https://doi.org/10.3389/fphar.2021.629695
- Mitrovic T, Stamenkovic S, Cvetkovic V, Radulovic N, Mladenovic M, Stankovic M, et al. Platismatia glauca and Pseudevernia furfuracea lichens as sources of antioxidant, antimicrobial, and antibiofilm agents. EXCLI J. 2014;13:938. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC4464406/
- Guvenc A, Akkol EK, Suntar I, Keles H, Yildiz S, Calss I. Biological activities of Pseudevernia furfuracea (L.) Zopf extracts and isolation of the active compounds. J Ethnopharmacol. 2012;144(3):726-34. Available from: https://doi.org/10.1016/j.jep.2012.10.021
- Kello M, Goga M. Lichen, Pseudevernia furfuracea (L.) Zopf: analytical compositional features, biological activity, and use in cancer studies. In: Ancient and Traditional Foods, Plants, Herbs, and Spices used in Cancer. CRC Press; 2023;281-96. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003260028-23/lichen-pseudevernia-furfuracea-zopf-martin-kello-michal-goga
- Sarikurkcu C, Kocak MS, Calapoglu M, Ocal C, Tepe B. Biological and phytochemical evaluation: Pseudevernia furfuracea as an alternative multifunctional agent. J Funct Foods. 2016;24:11-7. Available from: https://doi.org/10.1016/j.jff.2016.03.022
- Aoussar N, Manzali R, Nattah I, Rhallabi N, Vasiljevic P, Bouksaim M, et al. Chemical composition and antioxidant activity of two lichen species (Pseudevernia furfuracea L. and Evernia prunastri L) collected from Morocco. JMES. 2017;8(6):1968-76. https://www.jmaterenvironsci.com/Document/vol8/vol8_N6/209-JMES-3212-Aoussar.pdf
- Kello M, Kuruc T, Petrova K, Goga M, Michalova Z, Coma M, et al. Pro-apoptotic potential of Pseudevernia furfuracea (L.) Zopf extracted and isolated physodic acid in an acute lymphoblastic leukemia model in vitro. Pharmaceutics. 2021;13(12):2173. Available from: https://doi.org/10.3390/pharmaceutics13122173
- Joulain D, Tabacchi R. Lichen extracts as raw materials in perfumery. Part 2: treemoss. Flavour Fragrance J. 2009;24(3):105-16. Available from: https://doi.org/10.1002/ffj.1923
- Seklic DS, Jovanovic MM, Virijevic KD, Grujic JN, Zivanovic MN, Markovic SD. Pseudevernia furfuracea inhibits migration and invasion of colorectal carcinoma cell lines. J Ethnopharmacol. 2022;284:114758. Available from: https://doi.org/10.1016/j.jep.2021.114758
- Petrova K, Kello M, Kuruc T, Backorova M, Petrovova E, Vilkova M, et al. Potential effect of Pseudevernia furfuracea (L.) Zopf extract and metabolite physodic acid on tumour microenvironment modulation in MCF-10A cells. Biomolecules. 2021;11(3):420. Available from: https://doi.org/10.3390/biom11030420
- Bakir TO, Geyikoglu F, Colak S, Turkez H, Aslan A, Bakir M. The effects of Cetraria islandica and Pseudevernia furfuracea extracts in normal and diabetic rats. Toxicol Ind Health. 2015;31(12):1304-17. Available from: https://doi.org/10.1177/0748233713475521
- Essadki Y, Hilmi A, Cascajosa-Lira A, Girao M, Darrag EM, Martins R, et al. In Vitro Antimicrobial Activity of Volatile Compounds from the Lichen Pseudevernia furfuracea (L.) Zopf. Against Multidrug-Resistant Bacteria and Fish Pathogens. Microorganisms. 2024;12(11):2336. Available from: https://doi.org/10.3390/microorganisms12112336
- Karabulut G, Ozturk Sule. Antifungal activity of Evernia prunastri, Parmelia sulcata, Pseudevernia furfuracea var. furfuracea. Pakistan J Bot. 2015;47(4):1575-9. Available from: https://www.researchgate.net/publication/282712733_Antifungal_activity_of_Evernia_prunastri_Parmelia_sulcata_Pseudevernia_furfuracea_var_Furfuracea
- Calchera A, Dal Grande F, Bode HB, Schmitt I. Biosynthetic gene content of the ‘perfume lichens’ Evernia prunastri and Pseudevernia furfuracea. Molecules. 2019;24(1):203. Available from: https://doi.org/10.3390/molecules24010203
- Alom S, Ali F, Kakoti BB, Choudhury S, Ahmed AB. Lichen is a raw material in the perfumery and cosmetic industries. In: Chemistry, Biology, and Pharmacology of Lichen. 2024;275-87. Available from: https://doi.org/10.1002/9781394190706.ch17
- Incerti G, Cecconi E, Capozzi F, Adamo P, Bargagli R, Benesperi R, et al. Infraspecific variability in baseline element composition of the epiphytic lichen Pseudevernia furfuracea in remote areas: implications for biomonitoring of air pollution. Environ Sci Pollut Res. 2017;24(9):8004-16. Available from: https://link.springer.com/article/10.1007/s11356-017-8486-7
- Vingiani S, Adamo P, Giordano S. Sulphur, nitrogen and carbon content of Sphagnum capillifolium and Pseudevernia furfuracea exposed in bags in the Naples urban area. Environ Pollut. 2004;129(1):145-58. Available from: https://doi.org/10.1016/j.envpol.2003.09.016
- Vardar C, Basaran E, Cansaran-Duman D, Aras S. Air-quality biomonitoring: assessment of genotoxicity of air pollution in the province of Kayseri (Central Anatolia) by use of the lichen Pseudevernia furfuracea (L.) Zopf and amplified fragment-length polymorphism markers. Mutat Res Genet Toxicol Environ Mutagen. 2014;759:43-50. Available from: https://doi.org/10.1016/j.mrgentox.2013.09.011
- Demkova L, Bobul’ska L, Arvay J, Jezny T, Ducsay L. Biomonitoring of heavy metals contamination by mosses and lichens around Slovinky tailing pond (Slovakia). J Environ Sci Health A. 2017;52(1):30-6. Available from: https://doi.org/10.1080/10934529.2016.1221220
- Zesch, S., Panzer, S., Paladin, A., Sutherland, M. L., Lindauer, S., Friedrich, R., & Rosendahl, W. (2024). The multifaceted nature of Egyptian mummification: Paleoradiological insights into child mummies. Plos one, 19(12), e0316018. Available from: https://doi.org/10.1371/journal.pone.0316018
- Hurley K. Lichenpedia: A Brief Compendium. Vol. 11. Princeton University Press; 2024. Available from: https://www.degruyterbrill.com/document/doi/10.1515/9780691239897/html?lang=en&srsltid=AfmBOorDhUl23IF5rW0aV6Zx3fNfGYtZ8xkOL_UgjkkhsHnw7tJHdtYS
- Jacob I, Jacob W. The Healing Past: Pharmaceuticals in the Biblical and Rabbinic World. Brill, 1993. Available from: https://archive.org/details/healingpastpharm0000unse
- Seaton B. The Language of Flowers: A History. University of Virginia Press, 2012. Available from: https://www.upress.virginia.edu/title/2776/
- Arihilam NH, Arihilam EC. Impact and control of anthropogenic pollution on the ecosystem–a review. J Biosci Biotechnol Discov. 2019;4(3):54-9. Available from: https://doi.org/10.31248/JBBD2019.098
- Garty J. Biomonitoring atmospheric heavy metals with lichens: theory and application. Crit Rev Plant Sci. 2001;20(4):309-71. Available from: https://doi.org/10.1016/S0735-2689(01)80040-X?urlappend=%3Futm_source%3Dresearchgate
- Topal M, Arslan Topal EI, Obek E, Aslan A. Potential human health risks of toxic/harmful elements by consumption of Pseudevernia furfuracea. Int J Environ Health Res. 2022;32(9):1889-96. Available from: https://doi.org/10.1080/09603123.2021.1925635
- Tuncel SG, Yenisoy-Karakas S, Dogangun A. Determination of metal concentrations in lichen samples by inductively coupled plasma atomic emission spectroscopy technique after applying different digestion procedures. Talanta. 2004;63(2):273-7. Available from: https://doi.org/10.1016/j.talanta.2003.10.055