More Information
Submitted: June 16, 2025 | Approved: July 17, 2025 | Published: July 18, 2025
How to cite this article: Kharbyngar B, Rao SR. Phenotypic Characterisation of Rhizobial Strains Symbiotically Associated with Smithia ciliata and Desmodium polycarpum of Meghalaya. J Plant Sci Phytopathol. 2025; 9(2): 045-051. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001155
DOI: 10.29328/journal.jpsp.1001155
Copyright license: © 2025 Kharbyngar B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Desmodium polycarpum; Smithia ciliata; Rhizobia; Nodules
Phenotypic Characterisation of Rhizobial Strains Symbiotically Associated with Smithia ciliata and Desmodium polycarpum of Meghalaya
Banridor Kharbyngar1 and Satyawada Rama Rao2*
and Satyawada Rama Rao2*
1Bio-resources Development Centre (BRDC), Upper Shillong, Shillong-793009, Meghalaya, India
2Department of Life Science, School of Basic Sciences and Research, Sharda University, Greater Noida-201310, Uttar Pradesh, India
*Address for Correspondence: Satyawada Rama Rao, Professor, Department of Life Science, School of Basic Sciences and Research, Sharda University, Greater Noida-201310, Uttar Pradesh, India, Email: [email protected]
The present study focuses on the phenotypic characterisation of rhizobial strains isolated from the native legumes of Meghalaya. A total of seventy-five strains were isolated from various ecological regions, and sixteen strains from them were selected for further characterisation. It was observed that all sixteen rhizobial strains could not tolerate an alkaline pH value of 11. The majority of the fast-growing and slow-growing rhizobial strains in this investigation were found to have the highest temperature tolerance up to 40 ºC. It was also observed that all sixteen strains showed tolerance to different NaCl concentrations, and in the intrinsic antibiotic resistance test, the antibiotic Ampicillin (A10) was found to be highly effective in restricting the growth of the isolates, with only seven out of seventeen isolates being susceptible to this antibiotic. A total of eleven RNB isolates were found to be alkali-producing. It was observed that the rhizobial strain, NEHU-DP23, has the maximum number of fourteen sugars that were being utilised. The results revealed considerable phenotypic variability among the strains, indicating a broad adaptability to different environmental conditions.
Nitrogen, which is a component of many biomolecules, is considered one of the most widely distributed essential elements in nature [1]. Nitrogen, being a vital macronutrient, is the most crucial nutrient required for the growth and development of plants [2]. Biological nitrogen fixation (BNF) is a fundamental part of the nitrogen cycle that accounts for a massive portion of N available for plant uptake and significantly contributes to fixing nitrogen in terrestrial ecosystems [3]. During the past few decades, an enormous amount of nitrogen fertiliser has been employed to meet the needs of global food production [4]. As a result of excessive N application leads to several environmental and ecological problems [5], such as air pollution, soil pollution, and eutrophication of water bodies [6]. Therefore, it would be beneficial to study legume-rhizobia symbiosis that are suited to withstand environmental conditions like drought, high temperature, a wide range of soil pH, and salinity to select inoculants that would be able to endure and contribute to nitrogen fixation [7]. Temperatures above or below the ideal range of 25 °C - 30 °C, which is conducive to rhizobia, hurt legume nodulation and nitrogen fixation [8]. Numerous phenotypic traits have been used to recognise and distinguish between bacteria capable of nodulating legumes [9]. Based on their generation time and pH reaction on YEMA medium containing bromothymol blue, rhizobia were subsequently divided into two groups: the fast-growing, acid-producing group (Rhizobium sp.) and the slow-growing, alkaline-producing group, like Bradyrhizobium [10,11]. Therefore, screening of rhizobial strains that can withstand high temperature, with a wide range of soil pH, would be beneficial to use as inoculum, especially in regions with acidic soil of Meghalaya.
The genus Desmodium is distributed mainly in the tropical and subtropical regions of the world [12]. An interesting fact about this plant is that it can grow in low-fertility soils, with drought tolerance, hence making this plant a valuable representative in plant genetic resources, adding a significant contribution to forage production, soil protection, and improvement in small farming systems [13]. Smithia ciliata is an annual and diffuse herb [14] belonging to the family Fabaceae. It grows on the sloppy hills during rainy seasons, is native to the Himalayas, tropical and subtropical Asia, and is commonly known as fringed Smithia. In India, it is mainly found in Assam, Meghalaya, and Mizoram. However, few studies have been conducted on the identification and characterisation of stress-tolerant rhizobia from various native legumes found in the state of Meghalaya [15].
In the present study, we aimed to isolate rhizobia that are symbiotically associated with the native legumes, viz., D. polycarpum and S. ciliata of Meghalaya. Rhizobial strains were characterised at the phenotypic level to screen strains with tolerance to high salt concentration, temperature, pH, as well as their metabolic activities. Such a screening test assists in identifying beneficial and effective rhizobia that promote and improve agricultural productivity under stressed environments.
Isolation of rhizobia
Legume plants, S. ciliata and D. polycarpum, were collected from different areas of the state. Details of sampling sites are given in Table 1. The plant materials were sent for their identification and obtaining of accession number to the Botanical Survey of India (BSI), Shillong. Fresh and healthy nodules from each plant were surface sterilised with 90% (v/v) alcohol and 0.1% (w/v) BavistinR. The sterilised nodules were crushed using a sterile scalpel. The root exudates obtained were streaked on Congo red-Yeast Extract Mannitol Agar medium (CR-YEMA) with pH 7 [10,11]. White, round, convex, raised colonies with entire margins were picked from the parent plates and purified through the quadrant streaking method. Purified rhizobial strains were maintained on YEMA petriplates at 28 °C and stored on YEMA slants at 4 °C.
| Table 1: Collection sites of leguminous plants along with their GPS coordinates. | |||||
| Sl. No. |
Place of Collection | GPS Co-ordinates | Region | ||
| Latitude | Longitude | Altitude(m) | |||
| 1 | Laitlyngkot | 25°26’N | 91°50’E | 1844 | East Khasi Hills District |
| 2 | Lum Rapleng | 25°28’N | 91°55’E | 1864 | |
| 3 | Mawkynrew | 25°26’N | 91°59’E | 1583 | |
| 4 | Mawryngkneng | 25°54’N | 92°01’E | 1434 | |
| 5 | Mylliem | 25°49’ N | 91°81’E | 1818 | |
| 6 | NEHU | 25°36’ N | 91°54’E | 1387 | |
| 7 | Sohiong | 25°29’ N | 91°45’E | 1790 | |
| 8 | Umphrup | 25°29’ N | 91°53’E | 1770 | |
| 9 | Kynshi | 25°31’ N | 91°33’E | 1534 | West Khasi Hills District |
| 10 | Mairang | 25°32’ N | 91°38’E | 1650 | |
| 11 | Markasa | 25°31’ N | 91°25’E | 1526 | |
| 12 | Mawthadraishan | 25°33’N | 91°24’E | 1691 | |
| 13 | Nongstoiñ | 25°32’N | 91°20’E | 1490 | |
| 14 | Ialong | 25°46’N | 96°26’ E | 1365 | Jaiñtia Hills District |
| 15 | Jowai | 25°44’N | 92°20’E | 1363 | |
| 16 | Khliehriat | 25°36’N | 92°36’E | 1126 | |
| 17 | Ummulong | 25°51’N | 92°15’E | 1334 | |
| 18 | Umbang | 25°40’N | 91°53’E | 948 | Ri-Bhoi District |
| 19 | Umsning | 25°43’N | 91°52’E | 721 | |
| 20 | Quinine | 25°49’N | 91°52’E | 544 | |
| 21 | Nongpoh | 25°50’N | 91°57’E | 567 | |
| 22 | Marngar | 25°53’N | 91°55’E | 530 | |
| 23 | Umling | 25°57’N | 91°51’E | 283 | |
| 24 | Khanapara | 26°11’N | 91°82’E | 69 | |
| 25 | Umroi | 25°42’N | 91°58’E | 855 | |
| 26 | Khliehumstem | 25°45’N | 92°50’ E | 902 | |
pH, temperature, and salt tolerance
The YEMA agar plates with different pH ranging from 4-11 by adjusting it using different pH buffers [16] were inoculated with 10μl aliquot of activated broth cultures. The plates were incubated at 28 ºC for 5-10 days. Their temperature tolerance was determined by streaking the strains onto YEMA plates and incubating at different temperatures, 30 °C, 35 °C, 40 °C, 45 °C, and 28 °C as a control in a BOD incubator for 4-8 days for bacterial growth. Similarly, their NaCl tolerance was tested by streaking on YEMA plates supplemented with different concentrations of NaCl ranging from 0.5% to 4% (w/v) of NaCl and kept in a BOD incubator at 28 °C for a 3-4 day incubation period.
Acidification and alkalinization
The ability of the Bradyrhizobium strains to produce acid or alkali on YEMA broth medium supplemented with 25 mg/l bromothymol blue (pH indicator) as described by Somasegaran and Hoben [17] and Sankhla, et al. [18] was carried out using Bromo thymol blue as a pH indicator and were incubated for 2-5 days at 28 °C - 30 °C in BOD incubator. The plates were observed for a change in colour to yellow (acidic reaction) or blue (alkaline reaction).
Intrinsic antibiotic resistance (IAR) test
The intrinsic antibiotic resistance pattern (IAR) test was done to study the antibiotic sensitivity of the selected root nodule bacteria isolates using Kirby-Bauer’s disc diffusion method [19]. The HiMedia antibiotic discs supplemented with different concentrations were aseptically placed on the plates, and the zone of inhibition was observed after incubation at 28 °C for 2-3 days.
Carbon (sugar) utilisation test
The ability of the Bradyrhizobium strains to utilise various sugars as their sole carbon source was tested using HiMedia sugar discs. A total of 21 sugars were tested. Andrade’s peptone water (HiMedia) was prepared as per the manufacturer’s instructions, autoclaved, and allowed to cool. A single disc corresponding to a specific sugar was aseptically added to each well. The sterile well-plates containing discs and the media were aseptically inoculated with an activated broth and incubated at 28 °C in the BOD incubator for 2-3 days. Observations were made after every 24 hours for a change of colour. If the test isolates can metabolise the carbohydrate, acids will be produced, thus lowering the pH of the medium, and this causes a change in the Andrade indicator from pale straw to pink (Figure 1).
Download Image
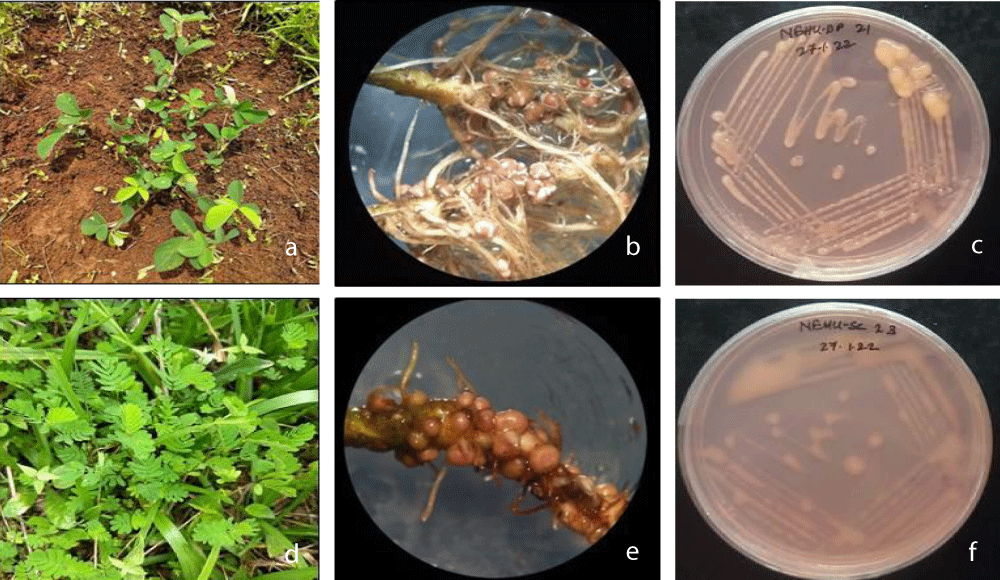
Figure 1: a) D. polycarpum plant (scale bar: 15 cm) b) Determinate type and have lenticels and are of desmodioid type of D. polycarpum (scale bar: 5mm). c. S. ciliata plant (scale bar: 15 cm)d. Determinate type, aeschynomenoid, do not have lenticels of S. ciliata (scale bar: 5mm).
Nodulation was observed in both the leguminous plants with a high number of nodules (Figure 2b,e). A total of seventy strains were isolated from the root nodules of D. polycarpum and S. ciliata, of which sixteen strains were selected for phenotypic characterisation. The tolerance of the selected isolates to different parameters such as pH, temperature, and salt concentrations was tested. The selected isolates were also analysed for their acidification and alkalinization properties, different sources of carbon utilisation, and their resistance to different antibiotic concentrations.
Download Image
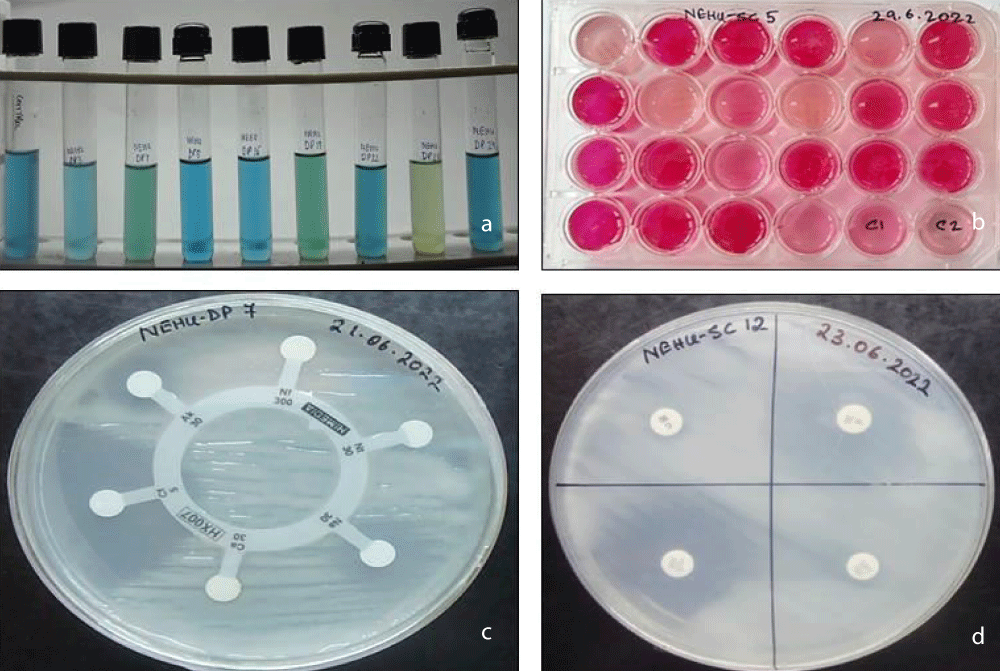
Figure 2: a. Acidification and alkalinization. b. Carbon utilization pattern. c. Intrinsic Antibiotic resistant pattern (Kirby’s method).
pH tolerance
Different pH values ranging from 4-11, supplemented with different buffers, were analysed to test the tolerance of the RNB isolates. It was observed that all sixteen rhizobial strains could not tolerate an alkaline pH value of 11. Most of the rhizobial strains, such as NEHU-DP7, NEHU-DP8, NEHU-SC6, NEHU-SC12, NEHU-SC13, NEHU-SC15, and NEHU-SC16, could tolerate up to a pH value between 4-10. However, it was observed that 9 strains, viz., NEHU-DP19, NEHU-DP22, NEHU-DP23, and NEHU-DP24 isolated from D. polycarpum and NEHU-SC8, NEHU-SC20, NEHU-SC21, NEHU-SC23, and NEHU-SC28, were able to survive at an acidic pH of 4 but could not tolerate an alkaline pH of 10 (Table 2).
| Table 2: Phenotypic characterization such as pH, T°C, NaCl tolerance, and acidification and alkalinisation response of selected RNB strains. | |||||
| Isolates | Location | pH tolerance | Temperature tolerance (ºC) | Salt tolerance (%) | Bromo Thymol Blue reaction |
| NEHU-DP 7 | Kynshi | 4-10 | 28-40 | 0.5-2 | Neutral |
| NEHU-DP 8 | Mawthadraishan | 4-10 | 28-35 | 0.5-1 | Alkalinisation |
| NEHU-DP 19 | Nongpoh | 4-7 | 28-40 | 0.5-2 | Neutral |
| NEHU-DP 22 | Marngar | 4-8 | 28-35 | 0.5-2 | Alkalinisation |
| NEHU-DP 23 | Umling | 4-7 | 28-40 | 0.5-2 | Acidification |
| NEHU-DP 24 | Khanapara | 4-7 | 28-40 | 0.5-2 | Alkalinisation |
| NEHU-SC 6 | NEHU | 4-10 | 28-35 | 0.5-2 | Neutral |
| NEHU-SC 8 | Sohiong | 4-8 | 28-35 | 0.5-1 | Alkalinisation |
| NEHU-SC 12 | Mawthadraishan | 4-10 | 28-35 | 0.5-1 | Alkalinisation |
| NEHU-SC 13 | Mawthadraishan | 4-10 | 28-40 | 0.5-2 | Alkalinisation |
| NEHU-SC 15 | Nongstoiñ | 4-10 | 28-40 | 0.5-3 | Alkalinisation |
| NEHU-SC 16 | Nongstoiñ | 4-10 | 28-40 | 0.5 | Neutral |
| NEHU-SC 20 | Khliehriat | 4-7.5 | 28-40 | 0.5-2 | Alkalinisation |
| NEHU-SC 21 | Khliehriat | 4-7.5 | 28-40 | 0.5 | Alkalinisation |
| NEHU-SC 23 | Umling | 4-7 | 28-40 | 0.5-2 | Alkalinisation |
| NEHU-SC 28 | Umroi | 4-9 | 28-40 | 0.5-3 | Alkalinisation |
Temperature tolerance
A total of sixteen rhizobial strains were being tested at different incubation temperatures from 28 ºC, 30ºC, 35 ºC, 40 ºC, and 45 ºC. It was observed that at the range between 28 ºC – 35 ºC, all sixteen isolates were able to show growth, out of which six isolates were isolated from D. polycarpum (NEHU-DP7, NEHU-DP8, NEHU-DP15, NEHU-DP19, NEHU-DP22, NEHU-DP23 and NEHU-DP24) and ten isolates were isolated from S. ciliata (NEHU-SC6, NEHU-SC8, NEHU-SC12, NEHU-SC13, NEHU-SC15, NEHU-SC16, NEHU-SC20, NEHU-SC21, NEHU-SC23 and NEHU-SC28).
In the present study, it was found that four rhizobial strains were from D. polycarpum, and seven rhizobial strains were found to have the highest temperature tolerance up to 40 ºC. At the range of 40 ºC, it was found that eleven strains, out of which four isolates, NEHU-DP7, NEHU-DP19, NEHU-DP23, and NEHU-DP24, were isolated from D. polycarpum, and seven isolates, NEHU-SC13, NEHU-SC15, NEHU-SC16, NEHU-SC20, NEHU-SC21, NEHU-SC23, and NEHU-SC28, isolated from S. ciliata, could tolerate this temperature. And 5 strains, i.e., NEHU-DP8 and NEHU-DP22 isolated from D. polycarpum and NEHU-SC6, NEHU-SC8, and NEHU-SC12 isolated from S. ciliata, could tolerate at a temperature only up to 35 ºC, and none were found to survive at a temperature of 45 ºC (Table 2).
Salt tolerance
Different concentrations of NaCl ranging from 0.5%, 1%, 2% and 3% were carried out to test the tolerance of the RNB isolates towards NaCl. It was observed that all sixteen strains showed tolerance to different NaCl concentrations. All sixteen RNB isolates were found to show tolerance at the concentration of 0.5%, out of which six rhizobial strains NEHU-DP7, NEHU-DP8, NEHU-DP19, NEHU-DP22, NEHU-DP23, and NEHU-DP24 were isolated from D. polycarpum, and eight RNB isolates, NEHU-SC6, NEHU-SC8, NEHU-SC12, NEHU-SC13, NEHU-15, NEHU-SC20, NEHU-SC23, and NEHU-SC28 isolated from S. ciliata.
In the present study, 14 strains were found to show tolerance up to 1% while only two isolates, NEHU-SC 16 and NEHU-SC21, showed tolerance only up to 0.5%. Further, it was also observed that out of these sixteen strains, eleven strains were able to tolerate up to a concentration of 2% and only 2 strains, NEHU-SC 15 and NEHU-SC28, could tolerate high salt concentrations of 3% (Table 2).
Acidification and alkalinization
This test was observed in response to a change in colour in yeast extract mannitol broth, supplemented with bromothymol blue dye (BTB). A total of eleven RNB isolates were found to be alkali-producing that including NEHU-DP8, NEHU-DP22, NEHU-DP24, NEHU-SC8, NEHU-SC12, NEHU-SC13, NEHU-SC15, NEHU-SC20, NEHU-SC21, NEHU-SC23, and NEHU-SC28. While only one strain, NEHU-DP23, was found to be acid-producing and the other four RNB isolates, NEHU-DP7, NEHU-DP19, NEHU-SC6, and NEHU-SC16, were found to be neutral (Table 2 and Figure 2a).
Intrinsic Antibiotic Resistance (IAR)
The pattern of Intrinsic Antibiotic Resistance (IAR) was measured in terms of the isolate’s sensitivity to antibiotics through the formation of a halo zone around the antibiotic discs (Himedia). In the present investigation, a total of twenty-three Hi-media antibiotic discs were used to test each strain’s resistance to different antibiotics individually. The sixteen strains displayed a wide range in their resistance to or sensitivity to different antibiotics. In our present study, we observed that the highest level of susceptibility for the antibiotics such as Gentamicin (Gen10), Ciproflaxin (Cf5), and Streptomycin (HLS300) was shown by all sixteen strains, and we found that the antibiotic Gentamicin (Gen10) showed the highest zone of inhibition for most of the strains. On the other hand, the highest the antibiotic Ampicillin (A10) was found to be highly effective in restricting the growth of the isolates with only seven out of seventeen isolates were susceptibility to this antibiotic level of resistant was found in Ampicillin (A10), Amoxyclav (Amc30), Imipenam (I10) and Nitrofurantoin (Nf300) and amongst them, (Table 3 and Figure 2c,d).
| Table 3: Intrinsic antibiotic resistance (IAR) pattern of selected rhizobial strains (Halozones measured in millimeters). | |||||||||||||||||
| Sl.no. | Antibiotics (Conc. in µg) |
NEHU-DP 7 | NEHU-DP 8 | NEHU-DP19 | NEHU-DP22 | NEHU-DP23 | NEHU-DP24 | NEHU-SC 6 | NEHU-SC 8 | NEHU-SC12 | NEHU-SC13 | NEHU-SC15 | NEHU-SC16 | NEHU-SC20 | NEHU-SC21 | NEHU-SC23 | NEHU-SC28 |
| 1. | Amikacin (Ak30) | 12 | 19 | 15 | 14 | 11 | 18 | 20 | 22 | 24 | 22 | 22 | 23 | 28 | 18 | 22 | 26 |
| 2. | Ampicillin (A10) | 0 | 5 | 0 | 6 | 0 | 0 | 0 | 5 | 5 | 6 | 0 | 0 | 0 | 6 | 7 | 0 |
| 3. | Amoxicillin (Amc30) | 0 | 8 | 6 | 8 | 0 | 9 | 6 | 10 | 6 | 9 | 0 | 7 | 8 | 0 | 6 | 8 |
| 4. | Augmentin (Au30) | 7 | 10 | 8 | 8 | 6 | 9 | 8 | 8 | 7 | 7 | 0 | 8 | 0 | 9 | 7 | 8 |
| 5. | Aztreonam (Ao30) | 10 | 12 | 14 | 8 | 9 | 13 | 0 | 12 | 10 | 10 | 0 | 14 | 8 | 0 | 14 | 7 |
| 6. | Ceftazidine (Ca30) | 8 | 0 | 8 | 6 | 9 | 8 | 0 | 9 | 13 | 0 | 0 | 8 | 8 | 7 | 8 | 0 |
| 7. | Ciproflaxin (C5) | 35 | 29 | 20 | 21 | 29 | 20 | 20 | 22 | 25 | 20 | 17 | 14 | 22 | 20 | 18 | 30 |
| 8. | Cefuroxime (Cxm30) | 10 | 8 | 11 | 13 | 10 | 11 | 14 | 12 | 10 | 10 | 14 | 15 | 8 | 12 | 7 | 11 |
| 9. | Co-trimoxazole (Cot25) | 10 | 16 | 9 | 9 | 10 | 8 | 10 | 12 | 15 | 10 | 0 | 14 | 12 | 8 | 11 | 12 |
| 10. | Cephalothin (Ch30) | 7 | 8 | 0 | 6 | 7 | 8 | 7 | 10 | 8 | 9 | 12 | 0 | 8 | 8 | 6 | 10 |
| 11. | Cephoxitin (Cn30) | 8 | 0 | 11 | 0 | 9 | 8 | 0 | 0 | 9 | 0 | 10 | 8 | 8 | 9 | 7 | 7 |
| 12. | Chloramphenicol (C30) | 10 | 12 | 7 | 8 | 10 | 7 | 7 | 9 | 8 | 7 | 10 | 8 | 10 | 10 | 9 | 7 |
| 13. | Gentamicin (Gen10) | 38 | 33 | 32 | 34 | 34 | 32 | 33 | 30 | 33 | 32 | 30 | 26 | 35 | 30 | 32 | 35 |
| 14. | Imipramine (I10) | 0 | 8 | 0 | 10 | 0 | 7 | 0 | 8 | 7 | 8 | 0 | 0 | 10 | 0 | 7 | 8 |
| 15. | Kanamycin (K30) | 8 | 12 | 17 | 20 | 12 | 18 | 14 | 23 | 21 | 24 | 20 | 25 | 17 | 20 | 36 | 20 |
| 16. | Netilin (Nt30) | 0 | 12 | 19 | 13 | 18 | 9 | 18 | 24 | 22 | 22 | 25 | 27 | 30 | 15 | 18 | 21 |
| 17. | Nalixidic acid (Na30) | 13 | 18 | 10 | 15 | 11 | 11 | 8 | 10 | 9 | 12 | 11 | 10 | 9 | 9 | 10 | 24 |
| 18. | Nitrofurantoin (Nf300) | 0 | 9 | 0 | 8 | 0 | 6 | 0 | 9 | 9 | 0 | 0 | 8 | 8 | 10 | 7 | 0 |
| 19. | Ofloxacin (Of5) | 11 | 0 | 0 | 12 | 10 | 12 | 12 | 15 | 12 | 10 | 15 | 11 | 12 | 13 | 10 | 15 |
| 20. | Piperacillin (Pc100) | 10 | 8 | 7 | 0 | 10 | 9 | 0 | 9 | 11 | 10 | 0 | 0 | 10 | 0 | 8 | 7 |
| 21. | Streptomycin (HLS300) | 24 | 27 | 24 | 25 | 25 | 28 | 29 | 26 | 27 | 26 | 28 | 23 | 25 | 28 | 35 | 30 |
| 22. | Spectinomycin (SPT100) | 11 | 10 | 8 | 8 | 7 | 10 | 10 | 9 | 7 | 10 | 11 | 8 | 11 | 9 | 9 | 7 |
| 23. | Tetracycline (Te30) | 15 | 13 | 13 | 11 | 12 | 13 | 12 | 12 | 11 | 13 | 12 | 13 | 0 | 0 | 8 | 0 |
Carbon utilisation pattern
A total of twenty-one sugars or carbon sources were being tested for their utilisation by the selected RNB isolates. It was observed that the rhizobial strain, NEHU-DP23, has the maximum number of fourteen sugars that were being utilised, followed by the isolates NEHU-DP22 and NEHU-SC12 that utilised eleven sugars and eight sugars, respectively, out of twenty-one sugars. However, the isolate NEHU-SC15 could utilise only four sugars, NEHU-SC6 could utilise only two sugars, and NEHU-DP7, NEHU-DP8, and NEHU-SC16 could utilise only one sugar. It was also observed that the isolates, NEHU-DP19, NEHU-SC13, NEHU-SC20, NEHU-SC21, NEHU-SC23, and NEHU-SC28 were not able to utilise all the twenty-one sugars as their carbon sources (Table 4 and Figure 2b).
| Table 4: Microbiology Sugar (Carbon) utilization pattern of selected rhizobial strains. | ||||||||||||||||
| Sugars | NEHU-DP 3 | NEHU-DP 7 | NEHU-DP 8 | NEHU-DP19 | NEHU-DP22 | NEHU-DP23 | NEHU-DP24 | NEHU-SC 6 | NEHU-SC12 | NEHU-SC13 | NEHU-SC15 | NEHU-SC16 | NEHU-SC20 | NEHU-SC21 | NEHU-SC23 | NEHU-SC28 |
| Adonitol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Arabinose | - | - | - | - | + | + | - | + | + | - | - | - | - | - | - | - |
| Cellobiose | + | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| Dextrose | + | - | - | - | + | + | - | - | - | - | + | - | - | - | - | - |
| Dulcitol | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fructose | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| Galactose | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - |
| Inositol | - | - | - | - | + | + | - | - | - | - | + | - | - | - | - | - |
| Inulin | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| Lactose | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
| Maltose | + | - | - | - | + | + | + | + | + | - | - | - | - | - | - | - |
| Mannitol | - | - | - | - | + | + | - | - | + | - | - | - | - | - | - | - |
| Mannose | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - |
| Melibiose | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Raffinose | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - |
| Rhamnose | - | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Salicin | - | - | + | - | - | + | - | - | + | - | + | + | - | - | - | - |
| Sorbitol | + | - | - | - | + | + | - | - | - | - | - | - | - | - | - | - |
| Sucrose | + | - | - | - | + | + | - | + | - | - | - | - | - | - | - | - |
| Trehalose | - | - | - | - | + | + | + | - | + | - | - | - | - | - | - | - |
| Xylose | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - |
The optimum pH for rhizobia growth is considered to be between 6.0 and 7.0 [9,20], and few rhizobia can thrive at pH values lower than 5.0 [21]. It has been reported that some mutants of rhizobium leguminosarum can grow at a pH as low as 4.5 [22], and another report was given by Graham, et al. 1994 [21] when they isolated Rhizobium tropici CIAT899 [21] from bean nodules that can grow on YEMA medium with pH 4.0. In the present study, all the tested bradyrhizobial strains were able to grow at the acidic pH 4.0, which suggests that the soil of this region is highly acidic. Similarly, Bradyrhizobium strains from India are also known to tolerate pH ranging from 4-10 [15,23]. The rhizobia strain’s ability to withstand acidity varies greatly. Rhizobia’s ability to survive in root nodules of leguminous plants is affected by soil acidity, rhizosphere, and soil persistence, especially in the tropical regions [24].
Temperature significantly affects the persistence and viability of rhizobial strains in soils. The majority of the fast-growing and slow-growing rhizobial strains in this investigation were found to have the highest temperature tolerance up to 40 ºC. Bradyrhizobium species capable of nodulating Desmodium incanum were isolated, characterised, and identified as B. yuanmingense and B. elkani that grow well at 40 ºC [12], although the ideal temperature range for the growth of rhizobial strains has been claimed to be 25 ºC - 30 ºC [25]. High temperature can hinder some types of rhizobia’s adhesion to the roots of legumes, the development of root hair, and the formation of infection threads. High temperature can also reduce the rate of nitrogenase activity, leghemoglobin synthesis, and enzyme activity in nodules [20] and also causes an increase in water loss through transpiration, which ultimately slows down nodulation and reduces rhizobial growth [26]. On the other hand, low temperatures can impair rhizobia viability by slowing or delaying the nodulation and nitrogen fixation processes [27].
The initial stages of the Rhizobium-legume symbiosis are inhibited by salt stress. Delgado [28] has studied the effects of salt stress on nodulation and nitrogen fixation of legumes. In the present study, the majority of the Bradyrhizobium strains could tolerate salt concentration up to 2%, while a few isolates could tolerate low concentrations of salt only up to 0.5% and some isolates could tolerate up to 3% of salt concentration. This finding is being supported by the report of Ojha, et al. 2017 [15] for the majority of the Bradyrhizobium strains isolated from E. chinense and F. vestita from Meghalaya that could tolerate up to 2% salt concentration. Some rhizobial strains can endure and tolerate more in saline soils than their legume host [29]. There are a few examples of salt-tolerant rhizobial species, such as Rhizobium fredii [30] and R. meliloti [31], while R. leguminosarum [26,32] is salt sensitive.
Antibiotic resistance is a historic and naturally occurring phenomenon that affects the ecosystem [33]. Antibiotic resistance has been frequently used in distinguishing the rhizobial strains and keeping track of their survival and occupancy in the root nodules of legume plants [34]. IAR (Intrinsic Antibiotic Resistance), which serves as one of the significant phenotypic markers, was also examined for the strains in the present investigation. In the present study, a large variation was observed in the intrinsic antibiotic resistance pattern for a variety of root nodulating bacteria, with Gentamicin was shown to have the highest level of susceptibility for the majority of the strains, followed by Ciproflaxin and Streptomycin, and the least susceptibility against most of the strains were observed to be resistant are Ampicillin and Nitrofurantoin. Therefore, this variation could be the main feature for horizontal gene transfer [35] from or to members of the same or different genera [36]. According to Cole and Elkan [36], R. japonicum (now B. japonicum) has additional chromosomal antibiotic resistance genes. The transfer of plasmid genes R factor to and between genetically marked strains of R. japonicum (now B. japonicum) was described by Kuykendall [37]. A similar report that supports this finding was also given by Beringer [38] when he found that the P group R factor could be transferable from E. coli K12 to R. leguminosarum and between R. leguminosarum strains.
Fast-growing rhizobia tend to consume a wide range of carbohydrates as compared to slow-growing bacteria [39]. In the present study, a huge variation was observed in the carbon utilisation pattern. Carbon sources such as dextrose, maltose, and salicin were being utilised by the highest number of strains, both slow and fast growers, followed by arabinose and trehalose, and mannose and sucrose. On the other hand, carbon sources like adonitol and dulcitol were unutilized by the selected strains, which are similar to the findings reported from microsymbionts isolated from native legumes of the same region [15].
- Wang ET, Tian CF, Chen WF, Young JP, Chen WX. Ecology and evolution of rhizobia. Singapore: Springer Singapore. 2019;23–39.
- Irfan M, Abbas M, Shah JA, Depar N. Contrasting response of wheat to one-time root zone fertilization of ordinary and polymer-coated urea for grain yield and nitrogen use efficiency. J Plant Nutr. 2022;45(11):1722–33. Available from: https://doi.org/10.1080/01904167.2021.2015382
- Davies‐Barnard T, Friedlingstein P. The global distribution of biological nitrogen fixation in terrestrial natural ecosystems. Glob Biogeochem Cycles. 2020;34(3):e2019GB006387. Available from: https://doi.org/10.1029/2019GB006387
- Sun J, Li W, Li C, Chang W, Zhang S, Zeng Y, et al. Effect of different rates of nitrogen fertilization on crop yield, soil properties, and leaf physiological attributes in banana under subtropical regions of China. Front Plant Sci. 2020 ;11:613760. Available from: https://doi.org/10.3389/fpls.2020.613760
- Song H, Huan W, Yuan G, Lu D, Chen X, Wang H. One-time root-zone nitrogen application increased wheat yield and nitrogen utilization under distinct planting row spacings in the Yangtze River Delta Region of China. Field Crops Res. 2023;295:108900. Available from: https://doi.org/10.1016/j.fcr.2023.108900
- Ren K, Xu M, Li R, Zheng L, Liu S, Reis S, et al. Optimizing nitrogen fertilizer use for more grain and less pollution. J Clean Prod. 2022;360:132180. Available from: https://doi.org/10.1016/j.jclepro.2022.132180
- Goyal RK, Habtewold JZ. Evaluation of legume–rhizobial symbiotic interactions beyond nitrogen fixation that help the host survival and diversification in hostile environments. Microorganisms. 2023;11(6):1454. Available from: https://doi.org/10.3390/microorganisms11061454
- Zhang F, Lynch DH, Smith DL. Impact of low root temperatures in soybean [Glycine max. (L.) Merr.] on nodulation and nitrogen fixation. Environ Exp Bot. 1995;35(3):279–85. Available from: https://doi.org/10.1016/0098-8472(95)00017-7
- Jordan DC. Family III, Rhizobiaceae. In: Bergey’s Manual of Systematic Bacteriology. 1984;1:235–44. Available from: https://www.scirp.org/reference/referencespapers?referenceid=2470441
- Vincent JM. A manual for the practical study of the root-nodule bacteria. International Biological Program; 1970. Available from: https://doi.org/10.1002/jobm.19720120524
- Somasegaran P, Hoben HJ. Methods in legume-Rhizobium technology. Paia, Maui: University of Hawaii NifTAL Project and MIRCEN, Department of Agronomy and Soil Science, Hawaii Institute of Tropical Agriculture and Human Resources, College of Tropical Agriculture and Human Resources; 1985. Available from: https://www.scirp.org/reference/referencespapers?referenceid=1657537
- Toniutti MA, Fornasero LV, Albicoro FJ, Martini MC, Draghi W, Alvarez F, et al. Nitrogen-fixing rhizobial strains isolated from Desmodium incanum DC in Argentina: Phylogeny, biodiversity, and symbiotic ability. Syst Appl Microbiol. 2017;40(5):297–307. Available from: https://doi.org/10.1016/j.syapm.2017.04.004
- Midega CA, Wasonga CJ, Hooper AM, Pickett JA, Khan ZR. Drought-tolerant Desmodium species effectively suppress parasitic Striga weed and improve cereal grain yields in western Kenya. Crop Prot. 2017;98:94–101. Available from: https://doi.org/10.1016/j.cropro.2017.03.018
- Bargali K, Bargali SS. Germination capacity of seeds of leguminous plants under water deficit conditions: implications for restoration of degraded lands in Kumaun Himalaya. Trop Ecol. 2016;57(3):445–53. Available from: https://www.researchgate.net/publication/287996592_Germination_capacity_of_seeds_of_leguminous_plants_under_water_deficit_conditions_Implication_for_restoration_of_degraded_lands_in_Kumaun_Himalaya
- Ojha A, Tak N, Rathi S, Chouhan B, Rao SR, Barik SK, et al. Molecular characterization of novel Bradyrhizobium strains nodulating Eriosema chinense and Flemingia vestita, important unexplored native legumes of the sub-Himalayan region (Meghalaya) of India. Syst Appl Microbiol. 2017;40(6):334–44. Available from: https://doi.org/10.1016/j.syapm.2017.06.003
- Howieson JG, Dilworth MJ. Working with rhizobia. Canberra: Australian Centre for International Agricultural Research; 2016. Available from: https://www.aciar.gov.au/sites/default/files/legacy/aciar_mn_173_web-updated_31_may_2016.pdf
- Somasegaran P, Hoben HJ. Handbook for rhizobia: methods in legume-Rhizobium technology. New York: Springer Science & Business Media; 2012. Available from: https://www.scirp.org/reference/referencespapers?referenceid=1881706
- Sankhla IS, Tak N, Meghwal RR, Choudhary S, Tak A, Rathi S, et al. Molecular characterization of nitrogen-fixing microsymbionts from root nodules of Vachellia (Acacia) jacquemontii, a native legume from the Thar Desert of India. Plant Soil. 2017;410:21–40. Available from: https://link.springer.com/article/10.1007/s11104-016-2838-9
- Cappucino JG, Sherman N. Microbiology: A Laboratory Manual. 7th ed. New York: Benjamin Cummings Pub. Co.; 2007. Available from: https://www.scirp.org/reference/referencespapers?referenceid=1183074
- Hungary M, Vargas MA. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res. 2000;65(2–3):151–64. Available from: https://doi.org/10.1016/S0378-4290(99)00084-2
- Graham PH, Draeger KJ, Ferrey ML, Conroy MJ, Hammer BE, Martinez E, et al. Acid pH tolerance in strains of Rhizobium and Bradyrhizobium, and initial studies on the basis for acid tolerance of Rhizobium tropici UMR1899. Can J Microbiol. 1994;40(3):198–207. Available from: https://doi.org/10.1139/m94-033
- Chen H, Richardson AE, Rolfe BG. Studies of the physiological and genetic basis of acid tolerance in Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1993;59(6):1798–804. Available from: https://doi.org/10.1128/aem.59.6.1798-1804.1993
- Rathi S, Tak N, Bissa G, Chouhan B, Ojha A, Adhikari D, et al. Selection of Bradyrhizobium or Ensifer symbionts by the native Indian caesalpinioid legume Chamaecrista pumila depends on soil pH and other edaphic and climatic factors. FEMS Microbiol Ecol. 2018;94(11):fiy180. Available from: https://doi.org/10.1093/femsec/fiy180
- Anyango B, Wilson KJ, Beynon JL, Giller KE. Diversity of rhizobia nodulating Phaseolus vulgaris L. in two Kenyan soils with contrasting pHs. Appl Environ Microbiol. 1995;61(11):4016–21. Available from: https://doi.org/10.1128/aem.61.11.4016-4021.1995
- Igiehon NO, Babalola OO, Aremu BR. Genomic insights into plant growth-promoting rhizobia capable of enhancing soybean germination under drought stress. BMC Microbiol. 2019;19:1–22. Available from: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-019-1536-1
- Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CL, Krishnamurthy L. Plant growth-promoting rhizobia: challenges and opportunities. 3 Biotech. 2015;5:355–77. Available from: https://doi.org/10.1007/s13205-014-0241-x
- Graham PH. Stress tolerance in Rhizobium and Bradyrhizobium, and nodulation under adverse soil conditions. Can J Microbiol. 1992;38(6):475–84. Available from: https://doi.org/10.1139/m92-079
- Delgado MJ, Ligero F, Lluch CL. Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean, and soybean plants. Soil Biol Biochem. 1994;26(3):371–6. Available from: https://doi.org/10.1016/0038-0717(94)90286-0
- Soussi M, Santamaria M, Ocana A, Lluch C. Effects of salinity on protein and lipopolysaccharide pattern in a salt‐tolerant strain of Mesorhizobium ciceri. J Appl Microbiol. 2001;90(3):476–81. Available from: https://doi.org/10.1046/j.1365-2672.2001.01269.x
- Yelton MM, Yang SS, Edie SA, Lim ST. Characterization of an effective salt-tolerant, fast-growing strain of Rhizobium japonicum. Microbiology. 1983;129(5):1537–47. Available from: https://doi.org/10.1099/00221287-129-5-1537
- Zhang X, Harper R, Karsisto M, Lindström K. Diversity of Rhizobium bacteria isolated from the root nodules of leguminous trees. Int J Syst Evol Microbiol. 1991;41(1):104–13. Available from: https://doi.org/10.1099/00207713-41-1-104
- Chien CT, Maundu J, Cavaness J, Dandurand LM, Orser CS. Characterization of salt-tolerant and salt-sensitive mutants of Rhizobium leguminosarum biovar viciae strain C1204b. FEMS Microbiol Lett. 1992;90(2):135–40.
- Xiong W, Sun Y, Zhang T, Ding X, Li Y, Wang M, et al. Antibiotics, antibiotic resistance genes, and bacterial community composition in freshwater aquaculture environment in China. Microb Ecol. 2015;70:425–32. Available from: https://doi.org/10.1007/s00248-015-0583-x
- Bromfield ES, Lewis DM, Barran LR. Cryptic plasmid and rifampin resistance in Rhizobium meliloti influencing nodulation competitiveness. J Bacteriol. 1985;164(1):410–3. Available from: https://doi.org/10.1128/jb.164.1.410-413.1985
- Van Hoek AH, Mevius D, Guerra B, Mullany P, Roberts AP, Aarts HJ. Acquired antibiotic resistance genes: an overview. Front Microbiol. 2011;2:203. Available from: https://doi.org/10.3389/fmicb.2011.00203
- Cole MA, Elkan GH. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973;4(3):248–53. Available from: https://doi.org/10.1128/aac.4.3.248
- Kuykendall LD. Transfer of R factors to and between genetically marked sublines of Rhizobium japonicum. Appl Environ Microbiol. 1979;37(5):862–6. Available from: https://doi.org/10.1128/aem.37.5.862-866.1979
- Beringer JE. R factor transfer in Rhizobium leguminosarum. Microbiology. 1974;84(1):188–98. Available from: https://doi.org/10.1099/00221287-84-1-188
- Graham PH, Parker CA. Diagnostic features in the characterisation of the root-nodule bacteria of legumes. Plant Soil. 1964:383–96. Available from: https://link.springer.com/article/10.1007/BF01373828