More Information
Submitted: February 20, 2025 | Approved: June 09, 2025 | Published: June 10, 2025
How to cite this article: Xolova M. Obtaining Synthetic Polyploid Sources through Hybridization of Diploid Species of Gossypium L. and Their Cytological Analysis. J Plant Sci Phytopathol. 2025; 9(2): 031-032. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001150
DOI: 10.29328/journal.jpsp.1001150
Copyright license: © 2025 Xolova M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gossypium L, G. anomalum; G. herbaceum; Synthetic polyploidy; Hybridization; Sporogenesis; Tetrad; Dyad; Cytogenetic analysis; Fertility
Obtaining Synthetic Polyploid Sources through Hybridization of Diploid Species of Gossypium L. and Their Cytological Analysis
Madina Xolova*
Westminster International University in Tashkent, Uzbekistan
*Address for Correspondence: Madina Xolova, Westminster International University in Tashkent, Uzbekistan, Email: [email protected]
The wild diploid species Gossypium anomalum is considered a promising genetic resource for improving the genetic traits of cultivated cotton varieties due to its natural adaptation to abiotic (e.g., drought, high temperatures) and biotic (e.g., insects, diseases) stress conditions. Due to the challenges in hybridizing cultivated tetraploid and wild diploid species, the genome of the F1 generation, obtained from the hybridization of wild species G. herbaceum subsp. pseudoarboreum (A1 genome) and Gossypium anomalum (B1 genome), was amplified through synthetic polyploidy to restore crossbreeding fertility. Cytogenetic analysis of F1С hybrids revealed unbalanced tetrads and abnormal dyads during sporogenesis. These anomalies, resulting from genetic differences between the species, negatively impacted the fertility of the hybrids. Additionally, disturbances in chromosome distribution during microsporogenesis were observed, leading to reduced viability of pollen grains in hybrid plants. Based on these findings, it was concluded that further breeding efforts are necessary to enhance the genetic stability and restore the fertility of these hybrids.
The wild diploid species Gossypium anomalum is a valuable genetic resource for improving the resilience of cultivated cotton varieties to various stress conditions. However, hybridization between cultivated tetraploid and wild diploid species is often challenging. To address this, synthetic polyploidy was employed to amplify the genome of F1 hybrids derived from G. herbaceum* subsp. pseudoarboreum (A1 genome) and G. anomalum (B1 genome). This study focuses on the cytogenetic analysis of these hybrids to understand the anomalies in sporogenesis and their impact on fertility [1].
The research was conducted from 2022 to 2025 at the Laboratory of Experimental Polyploidy and Phylogeny of Cotton, Institute of Genetics and Plant Experimental Biology, Uzbekistan Academy of Sciences, in Tashkent (41.369°N, 69.404°E, 480 m above sea level) [2,3]. The study involved G. anomalum, G. herbaceum subsp. pseudoarboreum, subsp. frutescens, and their polyploid hybrids: 27D F₁C (G. herbaceum subsp. pseudoarboreum × G. anomalum), 28D F₁C (G. herbaceum subsp. pseudoarboreum × G. anomalum), 26D F₁C (G. herbaceum subsp. pseudoarboreum × G. anomalum), and 31D F₁C (G. herbaceum subsp. frutescens × G. anomalum).
Plants were grown in Wagner pots under controlled conditions with a 16:8-hour photoperiod. Seeds were mechanically scarified and germinated in Petri dishes at 30 °C - 32 °C [4,5]. Seedlings were initially planted in a 1:1:1 mixture of compost, soil, and sand, and later transferred to Wagner pots. Polyploidy was induced by treating root tips with 0.2% colchicine solution for 24 hours in the dark. Hybridization was performed using standard methods. Cytological studies involved fixing collected anthers in Carnoy’s fixative (99.5% acetic acid and 96.0% ethanol) at 24 °C, staining with acetocarmine, and examining under a Leica DM750 microscope.
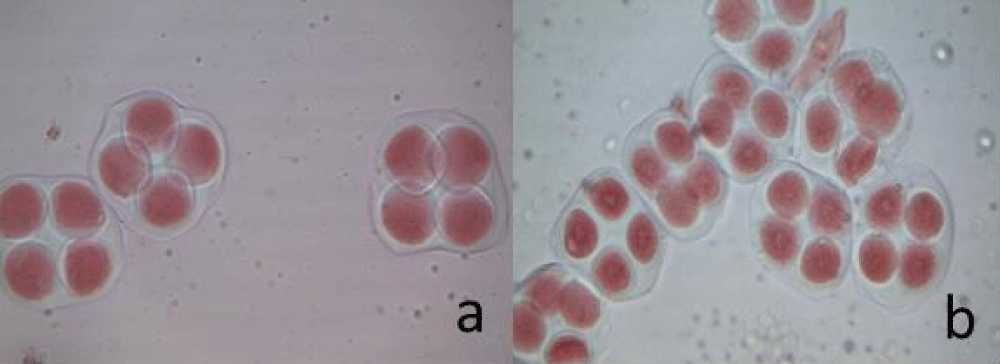
In the parental forms (G. herbaceum subsp pseudoarboreum and G. anomalum), balanced tetrads were predominantly observed during sporogenesis, with monads, dyads, and triads occurring less frequently, which is considered normal (Figure 1, Table 1).
Download Image
Figure 1: a) G. herbaceum subsp. pseudoarboreum b) Gossypium anomalum appearance of tetrads.
| Table 1: Meiotic Index and Micro-nucleated Tetrads in Studied Samples. | ||||
| No | Studied Samples | Total Sporads | Meiotic Index (%) | Micro-nucleated Tetrads (%) |
| 1 | G.anomalum | 838 | 99,76 ± 0,16 | - |
| 2 | G. herbaceum subsp.pseudoarboreum | 709 | 98,59 ± 0,44 | - |
| G.herbaceum subsp.frutescens | 803 | 96,66 ± 0,13 | - | |
| 4 | 28D F1C G.herbaceum subsp.pseudoarboreum × G.anomalum | 324 | 91,74 ± 1,52 | 0,80 ± 0,32 |
| 5 | 27D F1C G.herbaceum subsp.pseudoarboreum × G.anomalum | 164 | 45,50 ± 3,85 | 0,60 ± 0,60 |
| 6 | 26D F1C G.herbaceum subsp.pseudoarboreum × G.anomalum | 258 | 85,71 ± 2,17 | 1,53 ± 0,76 |
| 7 | 31D F1C G.herbaceum subsp.frutescens × G.anomalum | 788 | 91,27 ± 1,00 | 0,25 ± 0,17 |
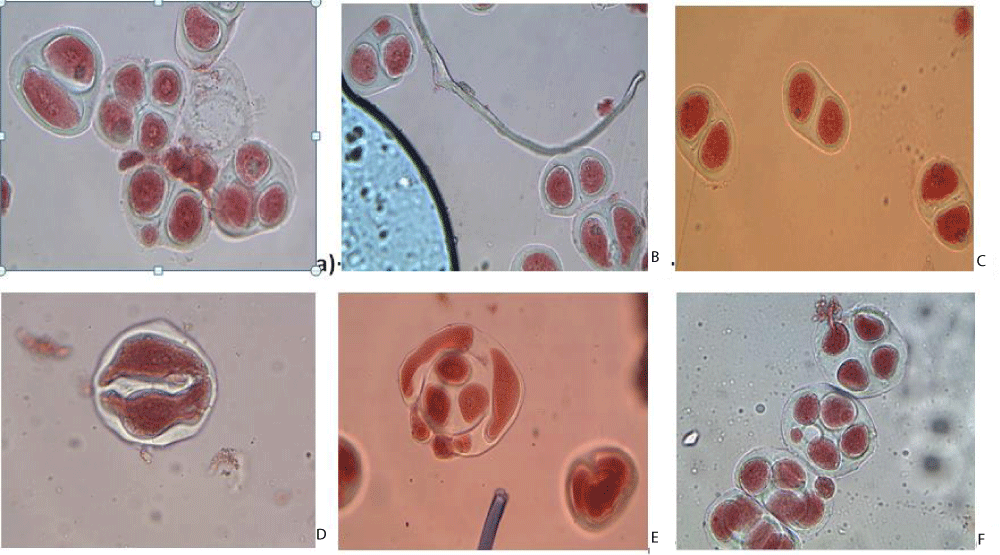
In the 27D F₁C hybrid (G. herbaceum subsp. pseudoarboreum × G. anomalum), dyads (45%) and normal tetrads (55%) were observed. In the 31D F₁C hybrid (G. herbaceum subsp. frutescens × G. anomalum), micro-nucleated dyads and tetrads (13%) and balanced tetrads (77%) were noted. These observations indicate unbalanced chromosome distribution and the formation of aneuploid chromosome sets (Figure 2).
Download Image
Figure 2: a), b), c), f) 27D F1C (G. herbaceum subsp. pseudoarboreum × G. anomalum); d), e) 31D F1C (G. herbaceum subsp. frutescens × G. anomalum).
Disturbances in meiosis led to the formation of polyads (1% - 2%) and unbalanced tetrads (5% - 15%). Despite the presence of unbalanced tetrads in the 31D F₁C hybrid, pollen grains were relatively well-formed [6,7] and the crossbreeding efficiency was higher.
- De Storme N, Geelen D. High temperatures alter cross-over distribution and induce male meiotic restitution in Arabidopsis thaliana. Commun Biol. 2020;3(1):187. Available from: https://doi.org/10.1038/s42003-020-0897-1
- Lei X, Ning Y, Eid Elesawi I, Yang K, Chen C, Wang C, Liu B. Heat stress interferes with chromosome segregation and cytokinesis during male meiosis in Arabidopsis thaliana. Plant Signal Behav. 2020;15(5):1746985. Available from: https://doi.org/10.1080/15592324.2020.1746985
- Yeung EC, Oinam GS, Yeung SS, Harry I. Anther, pollen and tapetum development in safflower, Carthamus tinctorius L. Sex Plant Reprod. 2011;24(4):307–317. Available from: https://doi.org/10.1007/s00497-011-0168-x
- Kaur K, Gupta RC, Kumari S. Cyto-morphological studies of some dicot plants from Rajasthan (India). Cytologia. 2015;80:353–362. Available from: http://dx.doi.org/10.1508/cytologia.80.353
- Russo G, Krauss M. Septin remodeling during mammalian cytokinesis. Front Cell Dev Biol. 2021;9:768309. Available from: https://doi.org/10.3389/fcell.2021.768309
- Jäger K, Fábián A, Barnabás B. Effect of water deficit and elevated temperature on pollen development of drought sensitive and tolerant winter wheat (Triticum aestivum L.) genotypes. Acta Biol Szeged. 2008;52:67–71. Available from: https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2583
- Pausheva ZP. Practical training in plant sitology. Method. Manual. Moscow: Agropromizdat; 1980.