More Information
Submitted: December 19, 2025 | Accepted: December 30, 2025 | Published: December 31, 2025
Citation: Lei Z, Yue Z, Yiran C, Yimeng C, Yi F. Advances in the Pathogenesis and Prevention of Apple Ring Rot. J Plant Sci Phytopathol. 2025; 9(3): 105-109. Available from:
https://dx.doi.org/10.29328/journal.jpsp.1001162
DOI: 10.29328/journal.jpsp.1001162
Copyright license: © 2025 Lei Z, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Apple; Apple ring rot; Pathogenesis; Disease resistance; Prevention and control strategies
Advances in the Pathogenesis and Prevention of Apple Ring Rot
Zou Lei1.2#, Zhang Yue2#, Chen Yiran1, Chen Yimeng1 and Feng Yi1*
1College of Horticulture, China Agricultural University, Beijing 100193, China
2Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China
#These authors contributed equally to this work
*Address for Correspondence: Feng Yi, College of Horticulture, China Agricultural University, Beijing 100193, China, Email: [email protected]
Apple ring rot disease severely restricts the development of the apple industry by impacting yield and quality. The primary pathogen, Botryosphaeria dothidea (Moug.) Ces. & De Not., causes widespread disease occurrence in major apple-producing regions. This paper describes disease symptoms, analyzes pathogenic and resistance mechanisms, and reviews prevention strategies. It identifies gaps in the current research and outlines future directions to support effective disease control and enable sustainable industry development.
Apple (Malus domestica), a globally dominant fruit crop, plays a pivotal role in global agricultural production and economies due to its extensive cultivation scale, industrial value, and market dynamics [1]. However, ring rot disease, caused by Botryosphaeria dothidea, is a highly destructive fungal disease, has increasingly threatened global apple production in recent years. This disease primarily infects branches and fruits throughout the growth cycle, causing wart-like lesions on branches that disrupt physiological functions and fruit rot that severely degrades fruit quality, thereby undermining the sustainability of the apple industry [1,2]. Geographically, apple ring rot is widespread across China’s major apple-producing regions, with higher incidence rates in extensively managed orchard systems. The expansion of cultivation areas and intensification of production have further exacerbated its prevalence [3]. Concurrently, rising food safety concerns and ecological awareness necessitate stricter disease management standards. While traditional chemical pesticides offer short-term control, their prolonged use risks pathogen resistance, disrupts orchard ecosystems, and compromises product safety [4]. Therefore, further investigation into the pathogenesis of ring rot and developing green, sustainable control strategies are critical for the industry’s long-term viability.
Symptomatic manifestations of Apple ring rot
Apple ring rot primarily infects branches and fruits of apple trees, and may occasionally spread to leaves. Infected leaves exhibit brown lesions with concentric rings.
Symptomatic manifestations on apple branches and trunks
At the early stage of infection of Botryosphaeria dothidea on apple branches and trunks, water-soaked brown spots emerge around lenticels, gradually expanding into reddish brown lesions (approximately 3–20 mm in diameter). These lesions develop a hard, raised center with a distinct ring groove separating them from healthy tissue, and the central area becomes progressively convex over time [5]. Lesions are widely distributed, affecting both large and small branches as well as trunks. When lesions encircle a branch or trunk, they disrupt nutrient and water transport, causing dieback of tissues above the lesion. Pruning and sawing wounds are primary infection sites for Botryosphaeria dothidea. Pathogens colonize necrotic cortex and inner xylem at wound sites, where some hyphae penetrate the protective cork layer to invade living cortical tissue. When branch resistance declines, pathogens in the living cortex proliferate rapidly, inducing canker lesions and subsequent branch dieback [6].
Symptomatic manifestations on apple fruits
Fruit infection typically develops during the enlargement-to-ripening stages. Early infection manifests as small, dark brown circular spots centered around lenticels. As the disease progresses, these spots expand outward, forming concentric ring-patterned rot lesions with alternating color bands. The lesions spread rapidly, often resulting in complete fruit rot within several days. Infected fruit emits a sour odor, and black pycnidia (spore-producing structures) appear on lesions in later stages. Under humid conditions, yellow mucilage may ooze from these lesions [7].
Pathogénique mechanism of Apple ring rot
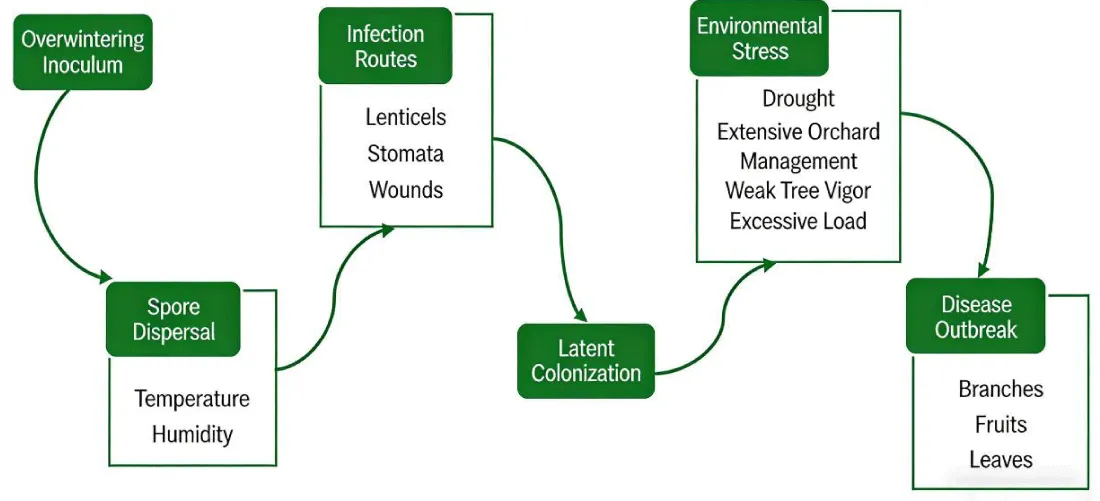
The pathogenic mechanism of apple ring rot involves the synergistic regulation of pathogen traits, virulence factors, and disease progression patterns. The disease develops through a dynamic “infection → factor activation → environmental induction” cascade. The pathogen’s infection cycle follows: overwintering inoculum, spore dispersal, infection routes → latent colonization → environmental stress → disease outbreak (Figure 1).
Etiological characteristics of apple ring rot
The pathogens causing apple ring rot are fungi, primarily Botryosphaeria dothidea, which is the dominant causative agent in apple-producing regions [8-10]. This pathogen produces two spore types: conidia (rain-dispersed) and ascospores (airborne). Upon landing on apple branches or fruits, spores germinate into germ tubes under suitable humidity and temperature conditions. The fungi initially grow saprophytically, absorbing nutrients from host surfaces to form reticular mycelium. When reaching lenticels, the mycelium utilizes nutrients from dead cells to develop cushion-like tissues or dense masses. Subsequently, highly infective hyphae differentiate and penetrate living tissues through intercellular spaces in the lenticel protective layer [5].
Botryosphaeria dothidea induces disease by secreting effector proteins, cell wall-degrading enzymes (CWDEs), toxins, and other virulence factors. Its pathogenic mechanism primarily involves effector protein functions: the Alt A1 effector enhances pathogenicity by inhibiting host cell death and immune responses, while gene knockout significantly reduces virulence [11]. CWDEs such as cellulase and pectinase degrade plant cell walls, facilitating hyphal expansion [12]. Ergot alkaloids, identified as novel virulence factors, promote infection by suppressing plant immunity [13]. Additionally, hypovirulent strains carrying dsRNA viruses (BdCV1/BdVV2) show reduced pathogenicity, suggesting viral regulation of the pathogenic process [10].
Disease development pattern of apple ring rot
The pathogens overwinter as mycelium or pycnidia in branch and trunk lesions. In spring, when temperatures exceed 15 °C and humidity reaches 80%, conidia disseminate via rain splash, initiating primary infection. Germinated spores invade host tissue through lenticels or wounds, where they remain latent and expand. Disease incidence typically peaks during fruit enlargement and branch dormancy. In Baishui County, Shaanxi Province, ring rot spore peaks occur from March to April and from August to October, coinciding with the rainy season, with maximum spore density observed at approximately 0.7 m above ground level [14]. In northern China’s apple-growing regions, from May–June, increased temperature and rainfall drive pathogen proliferation and spread, thereby marking a critical infection period [15]. Ring rot pathogens exhibit characteristics of latent infectio: after infecting branches and fruits, they remain dormant in tissues until favorable environmental conditions or weakened plant resistance triggers growth and disease outbreak [16].
Apple ring rot occurrence and progression are influenced by climatic conditions, field management, and cultivar resistance. High temperature and humidity promote pathogen growth, often exacerbating disease outbreaks. Poor orchard management—including inadequate fertilization, improper pruning, and poor ventilation—weakens tree vigor, reducing disease resistance and worsening rot severity [3]. For example, excessive nitrogen fertilization often increases fruit sugar content, favoring pathogen colonization, whereas potassium fertilization enhances resistance. Cultivars differ significantly in susceptibility: ‘Fuji’ and ‘Golden Delicious’ are highly susceptible, while ‘Ralls Janet’ and ‘Gala’ exhibit lower susceptibility [17]. The pathogen also infects pears and peaches, enabling cross-species transmission.
Disease resistance mechanism of apple against ring rot
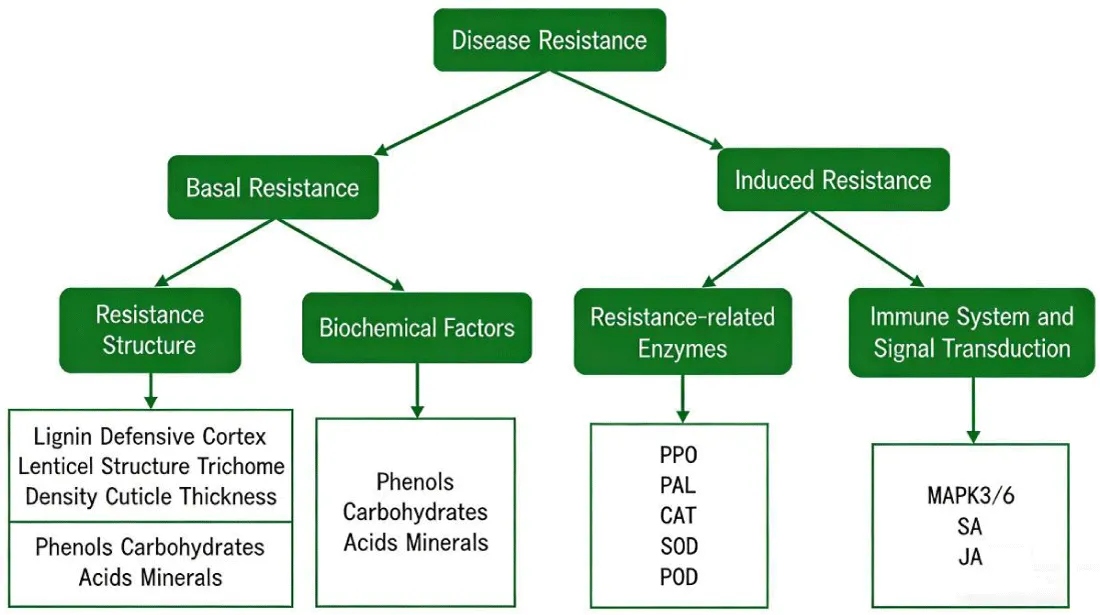
When infected by the ring rot pathogen, apple plants deploy a two-tier defense network comprising basal and induced resistance thereby establishing a core defensive barrier. Basal resistance derives from inherent structural and biochemical factors, forming the first line of defense against pathogen invasion. In contrast, induced resistance is a dynamic system activated by pathogen stimulation, triggering immune responses, resistance signaling pathways, and efficient defense gene expression (Figure 2).
Basal resistance of apple to ring rot
In plant defense against pathogens, lignin and defensive periderm serve as key structural resistance factors. Lignin, a structural polymer, accumulates extensively in cell walls, where it blocks the pathogen’s nutrient supply and neutralizes cell wall-degrading enzymes, thereby stabilizing cell wall structure [18]. When the ring rot pathogen invades apple branches and trunks, the host forms a defensive periderm comprising six to ten tightly arranged cell layers. This barrier physically isolates fungal hyphae from healthy cells, preventing pathogen diffusion and further infection [19]. Additionally, lenticel structure significantly influences apple disease resistance. Ultrastructural studies show that disease-resistant cultivars have smaller lenticels and thicker suberized cell layers compared to susceptible cultivars, effectively blocking pathogen entry [20]. Furthermore, trichome density and cuticle thickness are positively correlated with disease resistance in apples. For example, ‘Shennong No. 2’ exhibits higher trichome density on fruit surfaces than ‘Fuji’, resulting in fewer disease spots [21].
Apple basal resistance to ring rot depends on the synergistic effects of biochemical factors, including phenolic compounds, sugars, acids, and minerals, which collectively inhibit pathogen growth and proliferation [12]. During infection, these factors form a critical defense network. Mineral elements such as calcium (Ca), potassium (K), manganese (Mn), and nitrogen (N) further regulate resistance by influencing cell wall structure and metabolic processes. Specifically, Ca stabilizes biological membranes, regulates phenolic metabolism, and modulates cell wall permeability to inhibit pathogen hyphae. Increasing Ca in young apple fruits reduces respiration and energy consumption while promoting phenolic accumulation, thereby enhancing resistance. K thickens cell walls and facilitates cuticle development by promoting phenolic synthesis. Mn exhibits concentration-dependent effects, showing dual characteristics in infected apples. Conversely, N deficiency thins cell walls and reduces lignin content, facilitating pathogen infection [22].
Induced resistance of apple to ring rot
When pathogens infect plants, the plants activate pre-existing enzymes or induce new enzyme synthesis to initiate defense responses. These enzymes directly inhibit pathogen growth or catalyze the synthesis of resistance substances (e.g., lignin and phytoalexins), thereby constructing cellular protective structures. Enhanced activities of polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) are key indicators of both local and systemic disease resistance. They participate in the synthesis and metabolism of phenolic compounds and lignin, significantly improving plant defense [23]. PPO catalyzes enzymatic browning, promotes pigment production, and oxidizes phenolic compounds into toxic quinones, indirectly facilitating lignin biosynthesis and enhancing resistance. After inoculation with ring rot pathogens, PPO activity in apple cultivars varies, reflecting the role of phenolic metabolism in defense. PAL regulates the phenylpropanoid pathway, producing coumarins and flavonoids that convert into phytoalexins and lignin, strengthening fruit resistance. In the resistant cultivar ‘Jiguan’, PAL activity increases rapidly following inoculation, leading to extensive synthesis of chlorogenic acid, lignin, and phenolics, which thicken cell walls, inhibit pathogen expansion, and enhance resistance. Recent studies show that ring rot infection triggers the apple plant’s antioxidant system, generating enzymes like catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD). These enzymes scavenge reactive oxygen species (ROS), mitigating oxidative damage and improving disease resistance. In resistant cultivars, antioxidant enzyme activities surge shortly after inoculation, enabling efficient ROS clearance and pathogen growth inhibition [24].
Apple plants possess a sophisticated immune system that precisely identifies pathogens and initiates defense responses. The mitogen-activated protein kinase (MAPK) pathway is a key signal transduction event in plant innate immunity. For example, ring rot infection triggers MAPK3/6 phosphorylation in apples, significantly upregulating the WRKY33 transcription factor gene and promoting phytoalexin synthesis to resist pathogens [25]. Plant hormones such as salicylic acid (SA) are critical for immune responses [26]. Exogenous SA treatment enhances apple resistance to ring rot. SA interacts with the jasmonic acid (JA) pathway, and together they regulate defense responses. In resistant cultivars, pathogen inoculation rapidly increases SA and JA levels, with key synthesis genes showing higher expression than in susceptible cultivars [24].
Control strategies for Apple ring rot
Current approaches to apple ring rot focus on disease management through a comprehensive system combining agricultural, physical, chemical, and biological control strategies.
Agricultural control
Agricultural control is a cornerstone of apple ring rot prevention, enhancing tree vigor and disease resistance through orchard management. Key strategies include the following: (1) Fertilization: Prioritize organic and phosphorus-potassium fertilizers while limiting nitrogen to curb excessive growth. (2) Pruning: Ensure orchard ventilation and light penetration, and promptly remove diseased plant material to reduce pathogen spread. (3) Seedling management: Prevent pathogen transmission by avoiding cultivation near infected orchards and using disease-free stock for new plantings.
Physical control
Physical control methods help reduce pathogen populations, mitigating disease risk. Key techniques include: (1) Fruit bagging: Blocks pathogen infection and reduces fruit disease incidence. Bagging requires prior flower/fruit thinning to ensure uniform fruit quality. (2) Insect-proof nets: Covering orchards with nets helps exclude disease vectors (e.g., insects), curbing disease spread. (3) Branch and trunk scraping: Remove diseased tissue from branches and trunks, followed by prompt fungicide application to prevent reinfection.
Chemical control
Chemical control remains pivotal in preventing apple ring rot. During disease outbreaks, timely fungicide use can curb spread. Common fungicides include thiophanate-methyl, pyraclostrobin, and tebuconazole. Rational fungicide application is critical: select types and concentrations based on pathogen resistance monitoring to avoid overuse and resistance development. Compliance with usage standards ensures agricultural product quality and safety.
Biological control
Biological control is gaining attention because it is environmentally safe and sustainable. Biocontrol bacteria are a key strategy, enhancing plant resistance by competing for nutrients, secreting antimicrobial substances (e.g., lipopeptides), and inducing systemic resistance. This thereby jointly inhibiting the growth of apple ring rot pathogens and reproduction. Microbial metabolites are also critical, controlling ring rot by inhibiting spore germination or activating defense enzymes. Plant-derived fungicides, such as low-toxicity extracts, offer eco-friendly alternatives to chemical pesticides.
Prospects
Despite progress in apple ring rot research and control, several challenges persist. The pathogenic mechanism, particularly pathogen-host interactions, remains underexplored. Current prevention measures have curbed disease incidence but have limitations like high costs and inconsistent efficacy. Future research should primarily focus on: (1) Deepening understanding of pathogenicity and apple resistance mechanisms to support disease control theory. (2) Strengthening selection and promotion of disease-resistant cultivars to enhance overall apple resilience. (3) Developing novel biological control products/technologies to improve efficacy and stability. (4) Establishing a comprehensive control system, including an AI-driven precision pesticide application platforms, to enable green control and sustainable industry development.
- Zhang C, Yuan G, Han X, Li W, Cong P. Proteome analysis of different resistant apple cultivars in response to the stress of ring rot disease. Chinese Bulletin of Botany. 2020;55(4):430-441. Available from: https://www.chinbullbotany.com/EN/abstract/abstract60600.shtml
- Li Z, Chen Y, Ma Y. Research progress in apple ring rot: symptoms, pathogenesis, control measures, and perspectives. South China Fruits. 2025;:1-7.
- Hou M, Li Y, Jun G. Current status and cause analysis of apple canker in Changwu of the northern Weihe River. Journal of Agricultural Catastrophology. 2019;9(4):1-4.
- Wang L. Studies on fungicide resistance and screening of biocontrol strains of apple ring rot fungus Botryosphaeria dothidea [dissertation]. Wuhan: Huazhong Agricultural University; 2022.
- Li B. Histological mechanisms of infection and pathogenicity of apple ring rot and host disease resistance. In: Proceedings of the 2023 Annual Academic Conference of the Chinese Society of Plant Pathology; 2023.
- Liu K. Study on pathogen identification and control of dry rot in newly planted apple saplings [dissertation]. Yangling: Northwest A&F University; 2023.
- Li Y, Ma Y, Zeng H. Identification of a new pathogen of apple ring blight in Zhaotong. South China Fruits. 2024;53(6):203-209.
- Tang W, Ding Z, Zhou Q, Wang YZ, Guo LY. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Disease. 2012;96(4):486-496. Available from: https://doi.org/10.1094/PDIS-08-11-0635
- Liu Z, Lian S, Li B, Lu H, Dong X, Wang C. Draft genome sequence of Botryosphaeria dothidea, the pathogen of apple ring rot. Genome Announcements. 2016;4(5). Available from: https://doi.org/10.1128/genomea.01142-16
- Cong Q, Zhang J, Meng X, Dai PB, Li B, Hu TL, et al. Identification of hypovirus in apple ring rot fungus Botryosphaeria dothidea and detection of virus-carrying status in China. Scientia Agricultura Sinica. 2025;58(3):478-492. Available from: https://www.sciopen.com/article/10.3864/j.issn.0578-1752.2025.03.006
- Zhang H, Zhu X. Functional study of the Alt A1 effector protein of Botryosphaeria dothidea. In: Proceedings of the 2024 Annual Academic Conference of the Chinese Society of Plant Pathology; 2024.
- He X, He P, Chang Y. Advances in the research on resistance mechanism of apple to ring rot disease. Acta Horticulturae Sinica. 2020;47(8):1585-1594.
- Li Y, Gai Z, Wang C, Li P, Li B. Identification of mellein as a pathogenic substance of Botryosphaeria dothidea by UPLC-MS/MS analysis and phytotoxic bioassay. Journal of Agricultural and Food Chemistry. 2021;69(30):8471-8481. Available from: https://doi.org/10.1021/acs.jafc.1c03249
- Wei J. Characterisation of spatial and temporal distribution of three major apple pathogens in Shaanxi Province and construction of prediction model [dissertation]. Yangling: Northwest A&F University; 2024.
- Shi B. Development and utilization of a new protective agent for apple ring rot [dissertation]. Tai’an: Shandong Agricultural University; 2023.
- Han Z. Establishment and application of a detection method for the amount of Botryosphaeria dothidea carried in different parts of apple [dissertation]. Baoding: Hebei Agricultural University; 2021.
- Dong X, Zhang T. Occurrence pattern and systematic prevention and control of apple ring rot. Northwest Horticulture. 2021;(4):31-33.
- Martim DB, Brilhante A, Lima AR, Paixão DAA, Martins-Junior J, Kashiwagi FM, et al. Resolving the metabolism of monolignols and other lignin-related aromatic compounds in Xanthomonas citri. Nature Communications. 2024;15(1):7994. Available from: https://www.nature.com/articles/s41467-024-52367-6
- Han Q, Gao X, Wang J, Wang H, Huang L. Cytological and histological studies of the interaction between Botryosphaeria dothidea and apple twigs. Scientia Horticulturae. 2016;202:142-149. Available from: https://doi.org/10.1016/j.scienta.2016.03.002
- Xin L. Apple extracellular and intracellular infection mechanism of Botryosphaeria dothidea and a preliminary study of the resistance mechanism in apple cells [dissertation]. Tai’an: Shandong Agricultural University; 2022.
- Sun P, Shi Y, Zhang L, Li X, Ma Q, Li Z. Evaluation of apple and pear resistance to ring rot in Hohhot. Journal of Northern Agriculture. 2020;48(6):76-80. Available from: https://journal30.magtechjournal.com/bfnyxb/EN/abstract/abstract8994.shtml
- Cao Z. Study on the relationship between apple ring rot and deciduous disease and nutritive elements in apple trees [dissertation]. Tai’an: Shandong Agricultural University; 2018.
- Liu L, Li J, Qin W. Effect of salicylic acid on POD, PPO and PAL activities and fruit quality in Korla Xiangli pear. Xinjiang Agricultural Sciences. 2005;(2):98-101. Available from: https://www.xjnykx.com/EN/abstract/abstract3541.shtml
- Li Z. Study on the mechanism of resistance of different apple varieties to Botryosphaeria dothidea [dissertation]. Yantai: Yantai University; 2023.
- Qiu H. The molecular mechanism of ethylene-suppressed MAPK-WRKY33 immune signaling pathway induced by the apple ring rot pathogenic fungus Botryosphaeria dothidea [dissertation]. Tai’an: Shandong Agricultural University; 2017.
- Diaz-Vivancos P, Bernal-Vicente A, Cantabella D, Petri C, Hernández JA. Metabolomics and biochemical approaches link salicylic acid biosynthesis to cyanogenesis in peach plants. Plant and Cell Physiology. 2017;58(12):2057-2066. Available from: https://doi.org/10.1093/pcp/pcx135