More Information
Submitted: May 17, 2022 | Approved: June 13, 2022 | Published: June 14, 2022
How to cite this article: Asfaw MD. Chemical composition of olive stems essential oil from Ethiopia. J Plant Sci Phytopathol. 2022; 6: 057-061.
DOI: 10.29328/journal.jpsp.1001075
Copyright License: © 2022 Asfaw MD. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Olive; Essential oil; Dry distillation; Chemical composition; Olea Europaea
Chemical composition of olive stems essential oil from Ethiopia
Melese Damtew Asfaw*
Department of Chemistry, College of Natural and Computational Sciences, Mekdela Amba University, P.O. Box 32, Ethiopia
*Address for Correspondence: Melese Damtew Asfaw, Department of Chemistry, College of Natural and Computational Sciences, Mekdela Amba University, P.O. Box 32, Ethiopia, E-mail: [email protected]
In this article, the chemical compounds, antimicrobial and antioxidant activity of the volatile oil from leaves of Olea Europaea L. cultivar from Ethiopia has been studied. The essential oil was provided with a dry distillation apparatus and analyzed by GC-MS/FID. This analysis leads to the detection of 128 compounds representing 89.4% of the total oil. The major constituents were methyl ester hexadecanoic acid (4.10%), 2,4-dimethoxyphenolAa (4.05%), 2-methoxy-phenol (3.25%), 3,5-dimethoxy-4-hydroxytoluene (3.20%), 2-methoxy-5-methyl phenol (3.19%), 1,2,3-trimethoxy-5-methyl benzene (2.93%), 2-methoxy-4-vinyl phenol (2.70%), 2-hydroxy-3-methyl-2-cyclopenten-1-one (2.60%), trans-Isoeugenol (2.45%) and (E) -2,6-dimethoxy-4- (prop-1-en-1-yl) phenol (2.25%). The composition of essential oils was dominated by phenolic compounds.
Traditional medicine has been practiced in almost every culture, and it has spread worldwide and gained popularity [1]. In Ethiopia, knowledge of traditional medicine has been passed down from generation to generation, and about 80 percent of Ethiopians still rely on traditional medicine, especially for medicinal plants [2,3]. Essential oils are a complex mixture of variables commonly present in low concentrations and are essential components used for their flavor and aroma in the food, pharmaceutical, and perfume industries [4].
Olea Europaea commonly called wild olive is found throughout the Mediterranean, Europe, Africa, Iran, Asia, and Ethiopia and is thought to have a farming history of several 1000 years [5]. It holds historical significance in the religious context and is quoted in Christian and Hebrew Bibles and the Koran [5,6]. The olive tree is rarely eaten as a natural fruit because of its bitter taste but is used as oil or table olive and its wild and cultivated forms are considered an important subject of plant research [5]. O. Europaea has been shown in traditional medicine. It has been known to lower blood sugar, cholesterol, and uric acid. It is also used to treat diabetes, high blood pressure, inflammation, diarrhea, respiratory and urinary tract infections, gastrointestinal diseases, asthma, hemorrhoids, rheumatism, laxative, mouthwash, and vasodilator. Many phenolic compounds, especially secoiridoids and iridoids [7] and their pharmacological functions have been a major focus of scientists for the past decade [8,9]. However, the essential oil of Olea Europaea grown in Ethiopia has never been investigated before. Therefore, the purpose of this study was to determine the chemical composition of the essential oils Olea Europaea growing in Ethiopia through GC / MS analysis and to make comparisons with the literature.
Experimental
Description of the study area: Woreilu is one of the 24 administrative districts in the South Wollo Zone of Amhara Region, Ethiopia. It is located at 36° 26' 0" – 39° 43' 0" E longitude and 10° 34' 0" – 10° 60' 0 " N latitude and 492km far from Addis Ababa, Ethiopia, 571 km from Bahir Dar, the capital city of Amhara Region, as well as 91km from Dessie, West of Zonal town. As of the 2007 Ethiopia census, Woreilu town had a population of 14,817 and a 71013-hectare total area. According to the Agricultural and Rural Development office of the Woreda, agro-ecologically, the woreda is classified as “Dega” which accounts for 82% while the remaining 18% is “Woina Dega”. Of the total number of 23 kebeles administrations, 20 are rural. In the Woreda, most Kebeles produce crops in the “Meher” season, six kebeles in both seasons, and only one kebele in the “Belg” seasons. The agro-climatic conditions of the Woreda ranged from moderate to high, with an average altitude of 2730m above sea level. Annual rainfall ranges from 766.2 to 1250 mm. which is usually inadequate (short in duration), poorly distributed, and highly variable in inter and intra seasons.
Plant material
The dried olive stems were randomly collected from the local market of Woreillu town, South Wollo district, Ethiopia, in May 2018. The authenticity of the plant material was done in the Department of Biology and Biodiversity Management, Wollo University. The extraction of the Essential oil was employed by a traditional method (dry distillation) which is not previously been published Figure 1.
Figure 1: Dry pieces of Olea europaea stem (the author).
Isolation and characterization
The dried stems of Olea Europaera were cut (cut) into small pieces (≈20 cm long), weighed, and washed under tap water to remove any foreign material and dried on laboratory benches in a well-ventilated room before EO. About 2.0 kg of small pieces were loaded into a clay pot, after which, the pot was turned into a cooking pot (cooking pot) and well mixed (tightly sealed) with mud so that it would not emit any steam from it (outside). Finally, the packed jar was buried in a hole 50 to 50 inches [50 × 50 cm] in diameter and set fire to it. EO collection started after a temperature of about 30 minutes and lasted for 1 hour until the clay pot became red hot. The hot pot was cooled for 10 minutes as it was in the oven and the flexible EO collected due to evaporation of the stew pot was separated from the charcoal by burning and stored in 250 ml solid glass containers. Finally, EO was refrigerated until it was needed for chemical analysis and bioassays testing.
Stem EO analysis of Olea ertupaea was performed on a Shimadzu GC-2010 gas chromatograph with flame ionization detector (FID), inserted 25 m x 0.25 mm x 0.25 µm CBP5 capillary column, and using helium as a carrier gas. the oven temperature was set from 60 ºC (after 10 minutes) to 230 ºC at 3 ºC / min and the final temperature was 10 min. GC/MS analysis of stem EO of Olea ertupaea was performed with Agilent 5975N gas chromatograph-mass spectrometer with 30 m x 0.25 mm x 0.25 µm film thickness capillary column of HP5MS, using helium as a carrier gas. The oven temperature system was similar to that used in gas chromatography (GC) analysis.
The chemical properties of Essential Oils have been identified by comparing their MS with the reference spectra at the National Institute of Standards and Technology (NIST) mass spectrometry data center and by comparing their storage indicators with Kovats' indications in the literature. Quantitative data were obtained electronically at a percentage of the area and peaks combined without the use of a corrective factor [10].
It was noted that the local oil yield of Olea europaea was 315.5 mL obtained from 6kg of plant material in three abortion groups yielding a yield of 5.19 ± 0.05 (% v/w) (Table 1).
| Table 1: Percentage yield (% v/w) of Olea europaea EO. | |||
| Batch No. | Weight of plant material(kg) | Volume of Essential oil (mL) | Percentage yield(v/w) |
| 1 | 2.0 | 105 | 5.25 |
| 2 | 2.0 | 103 | 5.15 |
| 3 | 2.0 | 103.5 | 5.18 |
| Mean ± SD = 5.19 ± 0.05 | |||
The standard deviation of the three-group batch yield (% v/w) was found to be 0.05 equivalents to 0.96% of the standard deviation relative (% RSD). % Of RSD was used as an indicator of the accuracy of the dry distillation immersion process. The RSD% of this study, less than 2%, indicated that the dry distillation procedure was more accurate with less damage [11]. In addition, the distillation method used in this study yielded better results compared to other abortion techniques in classifying higher molecular terpenes such as diterpenes and triterpenes contradicting Birhanu's [12] study, which argued that diterpenes and higher terpenes cannot be detected by steam distillation method as these molecules are very heavy to allow evaporation, so they are rarely found in dissolved essential oils.
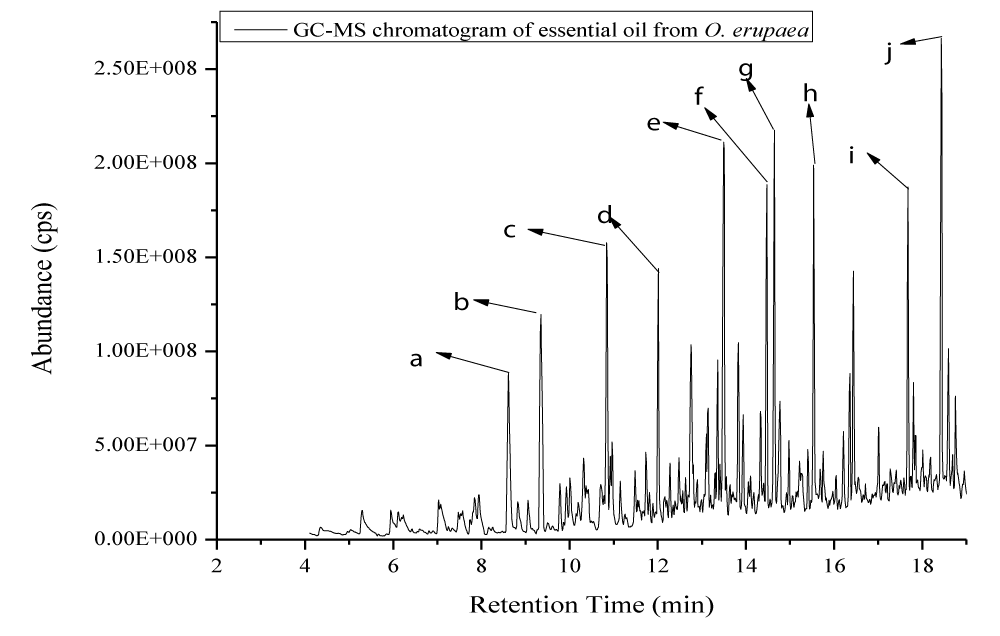
One hundred and twenty-eight compounds comprise 89.4% of the essential oils identified by GC and GC/MS. Its main compounds were methyl ester hexadecanoic acid, 2,4-dimethoxyphenol, 2-methoxy-phenol, 3,5-dimethoxy-4-hydroxytoluene, 2-methoxy-5-methyl phenol, 1,2,3-trimethoxy -5-methyl benzene, 2-methoxy-4-vinyl phenol, 2-hydroxy-3-methyl-2-cyclopenten-1-one, trans-Isoeugenol and (E)-2, 6-dimethoxy-4- (prop-1- en- 1-yl) phenol, respectively (Figure 2, Table 2). To the best of my knowledge, this is the first report on the production of essential oils from Ethiopian Olea europaea. Phenolic compounds (35.49%), non-terpenes (29.23%), terpenes (20.90%), and other compounds (6.37%) dominated fat formation.The essential oil of Olea europaea contains compounds of interesting biological properties. Some authors stated that phenolic compounds and their analogs have strong antibacterial, antifungal, antiviral, anti-mutagenic, anti-inflammatory, and antioxidant activities [13,14]. This could well explain the importance of the Olea europaea in the traditional Ethiopian pharmacopeia.
Figure 2: The representative ion chromatogram of the major compositions of the stem essential oil of Olea Europaea.
Table 2: The ten major compounds of the EO of the stem of Olea europaea. |
|||||||
| No | Name of compounds | Chemical formula | Retention time | Peaks | Area (%) | LRI* | Class |
| 1 | 2-hydroxy-3-methyl-2-cyclopen-1-one | C6H8O2 | 8.62 | a | 2.60 | 8.6178 | Ketone |
| 2 | 2-methoxy phenol | C7H8O2 | 9.35 | b | 3.25 | 9.3519 | Phenol |
| 3 | 2-methoxy-5-methylphenol | C8H10O2 | 10.85 | c | 3.19 | 10.851 | Phenol |
| 4 | 2-methoxy-4-vinylphenol | C9H10O2 | 12.76 | d | 2.70 | 12.763 | Phenol |
| 5 | 2,4-dimethoxyphenol | C8H10O3 | 13.50 | e | 4.05 | 13.503 | Phenol |
| 6 | trans-isoeugenol | C10H12O2 | 14.48 | f | 2.45 | 14.477 | Phenol |
| 7 | 3,5-dimethoxy-4-hydroxytolune | C9H12O3 | 14.65 | g | 3.20 | 9.3519 | Phenol |
| 8 | 1,2,3trimethoxy-5-methyl benzene | C10H14O3 | 15.54 | h | 2.93 | 15.545 | Benzene |
| 9 | (e)-2,6-dimethoxy-4-(prop-1-en-1-yl) phenol | C11H14O3 | 17.02 | i | 2.25 | 17.681 | Phenol |
| 10 | Methyl ester hexadecanoic acid | C17H24O2 | 18.45 | j | 4.10 | 18.44 | Fatty acid |
This investigation is different from those found in some oils from Algeria (from leaves) [15] (Palmetic acid, Z-nerolidol, Octacosane), Tunisia (from fruits and stem) [16], (3-ethyl pyridine, (E)-2-decanal, 2-ethylbenzaldehyde, and Nonanal, (E, E) -2,4-decenal, Benzyl alcohol respectively) and South Africa (from leaves) (Iweriebor, et al. 2012) (Nonanal, Phytol, 2-isopropyl-5-methyl-9methylenebicyclo[4.4.0]dec-1-ene. this variation in compositions and yield of the EO could be due to factors such as plant age, plant part, development stage, growing place, harvesting period, method of extraction, and principally by chemo-type since they influence the plant biosynthetic pathways and consequently the relative proportion of the main characteristic compounds [17] Table 3.
| Table 3: Chemical components of the stem oil of Olea europaea. | |||
| PK | Name of compounds | LRI | Area (%) |
| 1 | ethanedioic acid, bis(1-methyl propyl) ester | 4.3603 | 0.3862 |
| 2 | Silver butanoate | 4.9696 | 0.0485 |
| 3 | 3-Piperidinol, 1,4-dimethyl-, trans- | 5.0419 | 0.1902 |
| 4 | Pyrazole, 1,4-dimethyl- | 5.2979 | 0.7987 |
| 5 | 2-Furanmethanol | 5.9539 | 0.4980 |
| 6 | 1,6:2,3-Dianhydro-4-O-acetyl-.beta.-d-mannopyranose | 6.1198 | 0.3169 |
| 7 | 2,4-Pentanedione, 3-methyl- | 6.234 | 0.6179 |
| 8 | D-Limonene | 6.6053 | 0.2173 |
| 9 | 1,3-Cyclopentanedione | 7.0359 | 0.3313 |
| 10 | [1,3,4]thiadiazol, 2-amino-5-(2-piperidin-1-ylethyl)- | 7.0809 | 0.6856 |
| 11 | 2,5-Hexanedione | 7.2579 | 0.0920 |
| 12 | 2-Furancarboxaldehyde, 5-methyl- | 7.4851 | 0.2218 |
| 13 | Piperidine-4-carbonitrile | 7.5745 | 0.5887 |
| 14 | 2-Cyclopenten-1-one, 3-methyl- | 7.7484 | 0.1425 |
| 15 | tetrahydro[2,2']bifuranyl-5-one | 7.8526 | 0.6810 |
| 16 | 2(5H)-Furanone | 7.9479 | 0.7259 |
| 17 | 2(5H)-Furanone, 5-methyl- | 8.169 | 0.1052 |
| 18 | 2H-Pyran, 3,4-dihydro-2-methoxy- | 8.2595 | 0.1147 |
| 19 | 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 8.6178 | 2.5996 |
| 20 | 2-Furanone, 2,5-dihydro-3,5-dimethyl | 8.8347 | 0.5642 |
| 21 | Phenol | 9.0642 | 0.4353 |
| 22 | Phenol, 2-methoxy- | 9.3519 | 3.2458 |
| 23 | Methyl ethyl cyclopentene | 9.5105 | 0.1684 |
| 24 | Cyclohexane, (1-methylethylidene)- | 9.6202 | 0.0984 |
| 25 | Phenol, 2-methyl- | 9.7876 | 0.4697 |
| 26 | Cyclohexene, 1-methyl-4-(1-methylethyl)-, (R)- | 9.873 | 0.0718 |
| 27 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | 9.9341 | 0.4840 |
| 28 | Maltol | 10.016 | 0.7629 |
| 29 | Naphthalene | 10.205 | 0.5577 |
| 30 | Phenol, 3-methyl- | 10.326 | 0.9758 |
| 31 | Phenol, 2-methoxy-3-methyl- | 10.381 | 0.3767 |
| 32 | Oxirane, 3-hydroxypropyl- | 10.424 | 0.6297 |
| 33 | Glycoluril | 10.715 | 0.7149 |
| 34 | 2-Methoxy-5-methylphenol | 10.851 | 3.1932 |
| 35 | 2H-Azepin-2-one, hexahydro-1-methyl- | 10.931 | 0.5768 |
| 36 | Phenol, 2,4-dimethyl- | 10.972 | 0.8009 |
| 37 | 3,4-Dimethoxytoluene | 11.157 | 0.4976 |
| 38 | Phenol, 2,4,6-trimethyl- | 11.262 | 0.1204 |
| 39 | ethanone, 1-cyclohexyl- | 11.298 | 0.0839 |
| 40 | Phenol, 2-ethyl- | 11.491 | 0.5437 |
| 41 | Phenol, 4-ethyl- | 11.55 | 0.4447 |
| 42 | Benzene, 1-(2-butenyl)-2,3-dimethyl- | 11.664 | 0.1810 |
| 43 | 4-Hydroxy-2,4,5-trimethyl-2,5-cyclohexadien-1-one | 11.741 | 0.7174 |
| 44 | 2(3H)-Furanone, 5-acetyldihydro- | 11.816 | 0.2873 |
| 45 | Phenol, 2,4-dimethyl- | 11.896 | 0.1728 |
| 46 | 2-Pyridinealdoxime | 12.016 | 2.2458 |
| 47 | 2,4,6-Cycloheptatrien-1-one, 2-amino- | 12.163 | 0.5262 |
| 48 | Acetic acid,1-methyl-3-(1,3,3-trimethyl-bicyclo[4.1.0]hept-2-yl)- | 12.282 | 0.5426 |
| 49 | Naphthalene,1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1- | 12.353 | 0.1334 |
| 50 | 4-Hydroxy-3-methyl benzoic acid, methyl ester | 12.402 | 0.2437 |
| 51 | 1,4:3,6-Dianhydro-.alpha.-d-glucopyranose | 12.488 | 0.7493 |
| 52 | Cyclopentane, 2-methyl-1-methylene-3-(1-methylethenyl)- | 12.57 | 0.2661 |
| 53 | 2,4-Dimethylanisole | 12.638 | 0.3567 |
| 54 | 2-Methoxy-4-vinylphenol | 12.763 | 2.6978 |
| 55 | Pentadecane | 12.9 | 0.5852 |
| 56 | 4-ethylbenzoic acid, 2-(1-adamantyl)ethyl ester | 12.994 | 0.2205 |
| 57 | ethyl Vanillin | 13.142 | 1.7299 |
| 58 | Naphthalene, 2,6-dimethyl- | 13.204 | 0.2171 |
| 59 | Spirohexane-5-carboxylic acid, 1,1,2,2-tetramethyl-, methyl ester | 13.25 | 0.1113 |
| 60 | 5-Hydroxymethylfurfural | 13.304 | 0.4006 |
| 61 | Catechol | 13.361 | 1.2459 |
| 62 | Naphthalene, 2,6-dimethyl- | 13.419 | 0.3924 |
| 63 | 2,4-Dimethoxyphenol | 13.503 | 4.0507 |
| 64 | Benzene, 1,2,3-trimethoxy-5-methyl- | 13.562 | 0.2827 |
| 65 | ethanone, 1-(2,5-dimethoxyphenyl)- | 13.642 | 0.3037 |
| 66 | Aromandendrene | 13.714 | 0.3760 |
| 67 | Naphthalene, 1,2,3,4-tetrahydro-2,2,5,7-tetramethyl- | 13.761 | 0.1581 |
| 68 | 1,2-Benzenediol, 4-methyl- | 13.831 | 1.6478 |
| 69 | Phenol, 3,4-dimethoxy- | 13.941 | 0.9395 |
| 70 | 2(3H)-Furanone, 3-acetyldihydro-3-methyl- | 14.062 | 0.3197 |
| 71 | 1,4-Benzenediol, 2,5-dimethyl- | 14.109 | 0.3584 |
| 72 | 1,7-Octadien-3-one, 2-methyl-6-methylene- | 14.181 | 0.2387 |
| 73 | 1,2-Benzenediol, 3-methyl- | 14.339 | 1.1046 |
| 74 | Citral | 14.405 | 0.3183 |
| 75 | trans-Isoeugenol | 14.477 | 2.4466 |
| 76 | Methyleugenol | 14.553 | 0.1495 |
| 77 | 3,5-Dimethoxy-4-hydroxytoluene | 14.649 | 3.2041 |
| 78 | Benzaldehyde, 3-hydroxy-4-methoxy- | 14.774 | 1.4052 |
| 79 | m-ethylaminophenol | 14.927 | 0.1873 |
| 80 | ethanone, 1-(2,3,4-trihydroxyphenyl)- | 14.98 | 0.5293 |
| 81 | Benzene, 1-methyl-4-(methylsulfonyl)- | 15.065 | 0.1777 |
| 82 | 1,3-Benzenediol, 4,5-dimethyl- | 15.223 | 0.7862 |
| 83 | Naphthalene, 1,4,6-trimethyl- | 15.27 | 0.5307 |
| 84 | 3-Acetyl-2,5-dimethyl furan | 15.414 | 0.4634 |
| 85 | Benzene, 1,2,3-trimethoxy-5-methyl- | 15.545 | 2.9307 |
| 86 | Benzoic acid, 4-hydroxy-3-methoxy-, methyl ester | 15.693 | 0.4183 |
| 87 | ethanone, 1-[4-(methylthio)phenyl]- | 15.758 | 0.6582 |
| 88 | 5-Sec-butylpyrogallol | 15.975 | 0.3256 |
| 89 | Benzeneethanol, 4-hydroxy- | 16.049 | 0.2884 |
| 90 | Cyclohexanone, 2,5-dimethyl-2-(1-methylethenyl)- | 16.119 | 0.0865 |
| 91 | 3-tert-Butyl-4-hydroxyanisole | 16.217 | 0.6369 |
| 92 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | 16.36 | 1.0852 |
| 93 | 5,7-Dimethyl-1,3-diazaadamantan-6-one Hydrazone | 16.443 | 1.9776 |
| 94 | 1,4-Benzenediol, 2,3,5-trimethyl- | 16.556 | 0.5361 |
| 95 | 1,6-Dimethyl-4-ethylnaphthalene (Norcadalene) | 16.668 | 0.1184 |
| 96 | N',N'''-Bis(6-nitro-4H-pyran-2-ylmethylene)-2,5-pyridinedicarbohydrazide | 16.716 | 0.2386 |
| 97 | Dithiocarbonic acid,O-ethyl ester, methylene-S(IV)-trifluoromethyl est | 16.813 | 0.0660 |
| 98 | Phenol, 4-(3-hydroxy-1-propenyl)-2-methoxy- | 16.872 | 0.1100 |
| 99 | (e)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | 17.017 | 0.9346 |
| 100 | tyrosol, acetate | 17.151 | 0.4435 |
| 101 | 1-Acenaphthylenol, 1,2-dihydro-1-methyl- | 17.204 | 0.2000 |
| 102 | 1H-Cycloprop[e]azulen-4-ol,decahydro-1,1,4,7-tetramethyl-,[1aR | 17.286 | 0.4625 |
| 103 | 1,3-Oxathiolane, 2-(4-chlorophenyl)-2-methyl- | 17.413 | 0.5472 |
| 104 | 5-Methyl-5,8-dihydro-1,4-naphthoquinone | 17.518 | 0.4139 |
| 105 | Ketone, methyl 2-methyl-1-cyclohexen-1-yl, semicarbazone | 17.592 | 0.2294 |
| 106 | (e)-2,6-Dimethoxy-4-(prop-1-en-1-yl)phenol | 17.681 | 2.2473 |
| 107 | Benzenepropanol, 4-hydroxy-3-methoxy- | 17.745 | 0.2394 |
| 108 | 1,5,9-Undecatriene, 2,6,10-trimethyl-, (Z)- | 17.808 | 0.8683 |
| 109 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | 17.851 | 0.5235 |
| 110 | beta.-D-Mannofuranoside, farnesyl- | 17.895 | 0.2757 |
| 111 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | 18.019 | 0.6421 |
| 112 | 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl)-, | 18.076 | 0.3685 |
| 113 | 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl)-, | 18.188 | 0.6376 |
| 114 | tricyclo[4.3.0.0(7,9)]non-3-ene,2,2,5,5,8,8-hexamethyl-, | 18.324 | 0.5180 |
| 115 | Hexadecanoic acid, methyl ester | 18.44 | 4.1051 |
| 116 | 5,6-Azulenedimethanol,1,2,3,3a,8,8a-hexahydro-2,2,8-trimethyl- | 18.52 | 0.3135 |
| 117 | Naphthalene, 2,3-dimethoxy- | 18.598 | 1.3904 |
| 118 | Methyl 4-hydroxy-3,5-dimethoxybenzoate | 18.693 | 0.4963 |
| 119 | Benzaldehyde, 3,4,5-trimethoxy- | 18.761 | 1.1728 |
| 120 | 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene- | 18.96 | 0.4079 |
| 121 | Hexadecanenitrile | 19.082 | 0.5492 |
| 122 | 4-Hydroxy-2-methoxycinnamaldehyde | 19.144 | 0.1379 |
| 123 | Benzenepropanoic acid, 2,5-dimethoxy- | 19.215 | 1.1188 |
| 124 | 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl)-, | 19.3 | 0.5383 |
| 125 | Oxirane,2,2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-henei | 19.395 | 0.3530 |
| 126 | 7H-Furo[3,2-g][1]benzopyran-7-one, 4-hydroxy- | 19.492 | 0.1530 |
| 127 | .beta.-Humulene | 19.543 | 0.3816 |
| 128 | 3-Amino-7-methyl-1,2,4-benzotriazine 1,4-dioxide | 19.597 | 0.0695 |
| total | 89.395 | ||
The major components of the essential oil of the examined Olea europaea dry stems are methyl ester hexadecanoic acid, 2,4-dimethoxyphenol, 2-methoxy-phenol, 3,5-dimethoxy-4-hydroxytoluene, 2-methoxy-5-methyl phenol, 1,2,3-trimethoxy -5-methyl benzene, 2-methoxy-4-vinyl phenol. The essential oil of Olea europaea dry stems is a potential source of natural antioxidants and antibacterial compounds which are used for the treatment of various diseases caused by free radicals and microbes.
- van Galen E. Traditional herbal medicines worldwide, from reappraisal to assessment in Europe. J Ethnopharmacol. 2014 Dec 2;158 Pt B:498-502. doi: 10.1016/j.jep.2014.07.013. Epub 2014 Jul 17. PMID: 25043781.
- Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007 Apr 4;110(3):516-25. doi: 10.1016/j.jep.2006.10.011. Epub 2006 Oct 20. PMID: 17101251.
- Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop. J Health Dev. 2006; 20:127-134.
- Maffei ME, Gertsch J, Appendino G. Plant volatiles: production, function and pharmacology. Nat Prod Rep. 2011 Aug;28(8):1359-80. doi: 10.1039/c1np00021g. Epub 2011 Jun 13. PMID: 21670801.
- Masoko P, Makgapeetja DM. Antibacterial, antifungal and antioxidant activity of Olea africana against pathogenic yeast and nosocomial pathogens. BMC Complement Altern Med. 2015 Nov 17;15:409. doi: 10.1186/s12906-015-0941-8. PMID: 26577343; PMCID: PMC4650251.
- Muthee JK, Gakuya DW, Mbaria JM, Kareru PG, Mulei CM, Njonge FK. Ethnobotanical study of anthelmintic and other medicinal plants traditionally used in Loitoktok district of Kenya. J Ethnopharmacol. 2011 Apr 26;135(1):15-21. doi: 10.1016/j.jep.2011.02.005. Epub 2011 Feb 22. PMID: 21349318.
- Bendini A, Cerretani L, Carrasco-Pancorbo A, Gómez-Caravaca AM, Segura-Carretero A, Fernández-Gutiérrez A, Lercker G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules. 2007 Aug 6;12(8):1679-719. doi: 10.3390/12081679. PMID: 17960082; PMCID: PMC6149152.
- Ryan D, Robards K. Phenolic compounds in olives. Analyst. 1998; 123:31R-44R.
- Ghisalberti EL. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine. 1998 Apr;5(2):147-63. doi: 10.1016/S0944-7113(98)80012-3. PMID: 23195768.
- Lang G, Buchbauer G. A review on recent research results 2008-2010 on Essential oils as antimicrobials and antifungals, A review of Flavour and Fragrance Journal. 2011; 27(1):13-39.
- Caburian AB, Osi MO. Characterization and evaluation of antimicrobial activity of the Essential oil from the leaves of Piper betle L., International Scientific Research Journal. 2006. 2(1):2-13.
- Birhanu T. Chemical investigation on the Essential oil of Artimesia schimperi. Addis Ababa University, Ethiopia. 2012.
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67-103. doi: 10.1080/10408399209527581. PMID: 1290586.
- Silici S, Ünlü M, Vardar-Ünlü G. Antibacterial activity and phytochemical evidence for the plant origin of Turkish propolis from different regions. World J Microbiol Biotechnol. 2007 Dec;23(12):1797-803. doi: 10.1007/s11274-007-9430-7. Epub 2007 May 26. PMID: 27517836.
- Lograda T, Ramdani M, Chaker Adel N, Boukhebti H. Chemical and antimicrobial properties of Essential oils of Olea europea L. Laboratory of Natural Resource Valorisation, SNV Faculty, Setif 1 University, 19000 Setif, Algeria. 2015.
- Marzouk B, Haloui E, Fenina N, Bouftira I, Bouraoui A. Pharmacological activities and chemical composition of the Olea europaea L. leaf Essential oils from Tunisia. 2010.
- Viuda-Martos M, Mohamady MA, Fernández-López J, AbdelRazik KA, Omer EA, Pérez-Alvarez JA, Sendra E. Invitro antioxidant and antibacterial activities of Essentials oils obtained from Egyptian aromatic plants, Food Control. 2011; 22(1): 1715-1722.