More Information
Submitted: June 09, 2023 | Approved: July 01, 2023 | Published: July 03, 2023
How to cite this article: Pérez-Aguilar H, Lacruz-Asaro M, Arán-Ais F. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023; 7: 042-047.
DOI: 10.29328/journal.jpsp.1001104
Copyright License: © 2023 Pérez-Aguilar H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Amino acids; Enzymatic hydrolysis; Wastewater; Rendering; protein recovery
Abbreviations: PAPs: Processed Animal Proteins; ABP: Animal By-product; Cat: Category; AA: Amino Acids
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers
H Pérez-Aguilar*, M Lacruz-Asaro and F Arán-Ais
INESCOP, Footwear Technology Centre, Elda, Alicante, 03600, Spain
*Address for Correspondence: H Pérez-Aguilar, INESCOP, Footwear Technology Centre, Elda, Alicante, 03600, Spain, Email: [email protected]
Free amino acids-based biostimulants are gaining momentum in Europe for sustainable agriculture. They stimulate plant growth, improve crop productivity, and reduce reliance on harmful fertilizers. Enzymatic hydrolysis is used to develop biostimulants from animal by-products, such as greaves and protein-rich wastewater from processed animal proteins. The effectiveness of enzymatic hydrolysis depends on selecting the appropriate conditioning stage for the by-products, yielding protein in the range of 86% to 97%. These protein hydrolysates, with optimal amino acid compositions, are evaluated as biostimulants. Promising results show growth improvements of 17% to 31% in Chinese cabbage and lettuce seeds. The optimal dilution concentration ranges from 0.05% to 0.3%, depending on the protein hydrolysate used. The findings highlight the potential of these biostimulants to enhance plant growth and productivity while reducing environmental impact by replacing chemical fertilizers. They offer sustainable alternatives for promoting environmentally friendly practices in agriculture.
Modern agricultural practices and future perspectives for agroindustry are of current relevance and are faced with improving crop quality and yield as a result of the growing world population. Therefore, minimising the impact on the environment and human health caused by the widespread use of mineral fertilisers and chemical products intended for improving crop quality and yields must be prioritised [1]. In this context, the forthcoming regulatory framework is restricting the use of such chemical inputs due to the growing demand for organic food and environmental awareness. Biostimulants have the potential to mitigate these issues and provide a renewable option for improving crop quality and yield by simplifying the nitrogen life cycle in plants. In the conventional nitrogen life cycle, animal proteins (collagenous residue) are brought into contact with the soil (buried). Then, they encounter the bacterial population in the soil degrading the protein that produces organic nitrogen, which is then transformed into nitrates (nitrification). The nitrates are then absorbed by plant roots and converted into plant amino acids by the leaves, and finally, transformed into plant proteins, which are ingested by livestock, digested, and converted back into animal protein, thus closing the nitrogen life cycle.
Presently, only protein recovery from category 3 ABP such as liver, viscera, bone, intestines, and other by-products produced in slaughterhouses has been carried out [2-10] by means of enzymatic hydrolysis and recovery of protein by means of other applications [11-14]. In addition, a wide variety of studies have been carried out to obtain high-added-value products from tannery wastes. Studies have been carried out using alkaline, acid, and heat treatments [15,16] with good results for hydrolysed protein from tannery wastes. Nevertheless, depending on the application of the hydrolysed product such as food industry, retanning agent, biostimulants, texturisers, gelatine, or thickness agents, hydrolysis conditions are different [17-26] and different properties are required. Therefore, the recovery of category 2 by-products is very innovative as there are no studies that include this type of transformation to obtain protein biopolymers for agriculture applications. On the other hand, there is a patent lack of literature on the recovery of protein from poultry meal wastewater by enzymatic hydrolysis. In addition, only literature on wastewater treatments for protein separation such as ultrafiltration, isoelectric solubilization, electrocoagulation, etc. can be found [27-34]. Therefore, proteins obtained from enzymatic hydrolysis are a valuable source for organic agriculture and help close the loop of valuable resources. In addition, their recovery process contributes to the Sustainable Development Goals 6 and 12, of the United Nations (UN SDGs), preventing and reducing the management and disposal of wastewater and recovering it for bioprocesses with higher added value for the industry.
Against this background, biostimulants from animal by-products or biowaste by means of enzymatic hydrolysis have been developed as a more sustainable and versatile process using different slaughterhouse raw materials such as greaves and wastewater rich in protein from PAPs with a good protein recovery [35].
For the purpose of this work, two different types of raw material were recovered, namely, category 2 Processed Animal Proteins (PAPs) and category 3 wastewater. To recover these raw materials, a preparatory step, prior to the enzymatic hydrolysis process, was required as established by Regulation (EC) No 1069/2009, which is described hereafter.
Category 2 PAPs (PAP-1), which originate from pig carcases, were previously pressure-sterilised through method 1, with 50 mm particle size, at 133 °C for at least 20 minutes, without interruption, at an absolute pressure of at least 3 bar. This raw material was supplied by a rendering company (Energy Green Gas Almazán S.A., Almazán – Soria, Spain).
Category 3 wastewater is the condensed (protein-rich) water obtained from the drying of category 3 ABPs to produce Meat and Bone Meals (MBM). The sterilisation method used for category 3 ABPs was method 5 with 20 mm particle size, at 80ºC, for 120 minutes. Once the sterilisation was conducted, those ABPs could be utilised in PAPs. This raw material was supplied by a waste management company (Granja Otivar S.A., Alcalá de Guadaira – Sevilla, Spain).
Once the preparatory step was completed, enzymatic hydrolysis was conducted. This process requires rigorous control of temperature, pH, and hydrolysis time to obtain optimal enzyme activity. Nevertheless, due to the diversity in the composition of the raw materials used in this work, a conditioning step (defatting pre-treatment) was required to remove the high fat-content present in animal by-products and improve the efficiency of the enzymatic hydrolysis process to be carried out on the animal by-products.
The determination of total amino acids was carried out according to ISO 13903:2005 by derivatisation of the amino acids to convert them into chromophores. A liquid chromatographic separation was also conducted with a fluorescence detector and diode array detector in accordance with the previously mentioned standard. The determination of free amino acids was carried out according to ISO 13903:2005 with dilute hydrochloric acid 0.01N. The co-extracted nitrogenous macromolecules were precipitated with sulfosalicylic acid and removed by filtration.
This conditioning step consists of the characterisation of the raw materials used for the purposes of this work (i.e. PAPs and wastewater) in order to identify their composition and improve the process (Table 1). It must be noted that the final values considered as the results have been obtained from the average values after summing the results of each of the three times the tests were repeated.
| Table 1: Composition of raw materials category 3 wastewater and category 2 PAPs (greaves). | ||
| Parameters | Cat 3 Wastewater | Cat 2 PAP |
| pH | 6.29 ± 0.05 | 6.48 ± 0.05 |
| Solids content (%) | 19.49 ± 1.36 | 91.49 ± 1.44 |
| Moisture (%) | 80.51 ± 1.37 | 8.81 ± 1.11 |
| Fat content (% in dry matter) | 35.44 ± 0.82 | 39.95 ± 0.89 |
| Ash content (% in dry matter) | 6.84 ± 0.85 | 7.7 ± 0.91 |
| Total amino acids content (% in dry matter) | 40.31 ± 2.15 | 43.84 ± 2.29 |
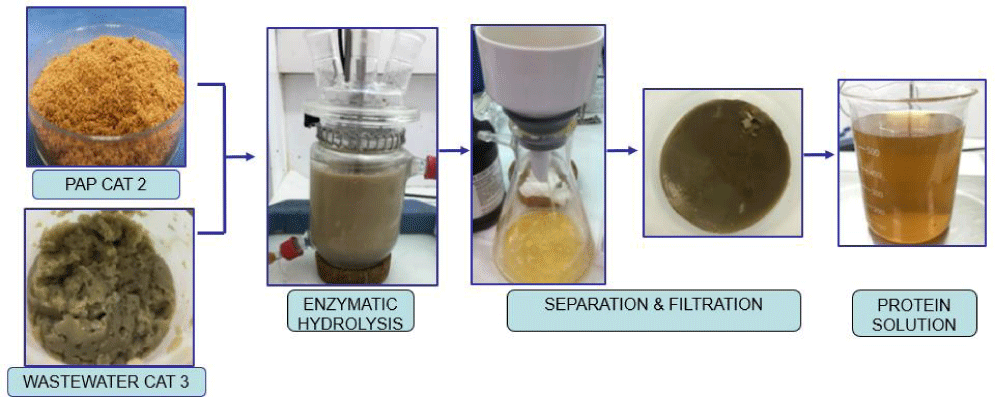
Subsequently, the experimental procedure of this research (i.e., enzymatic hydrolysis) was carried out following the process described hereafter and developed in [35,36] (Figure 1). In this procedure, different enzymes with endo- and exo-proteolytic activity, working at neutral (7) and alkaline (8-11) pH, were used to assess the degree of process yield, total amino acids and free amino acids content of the category 2 PAPs and category 3 wastewater evaluated. The solution obtained from enzymatic hydrolysis was then separated and filtered, which resulted in the achievement of the hydrolysed protein solution with optimal conditions.
Figure 1: Process to obtain hydrolysed protein from Category 2 PAPs and Category 3 wastewater.
Finally, a test to validate the protein solution as a biostimulant was carried out through the evaluation of growth by germination in a Petri dish according to standard ISO 16086-2:2012 and [35,36]. In this procedure, two different plant seed varieties [Chinese cabbage (Brassica rapa pekinensis) and lettuce (Lactuca Sativa)] were watered with 2 mL of different biostimulant concentrations, 0.01% - 0.3%, and left to grow at 24 ºC for 3 days. Then, water with 2 mL of biostimulant concentration was added again and the seeds were left to grow at 24 ºC for 3 more days. Finally, the germination length of the seeds in each Petri dish was measured and compared with the control sample. In this study, germinated seed lengths of less than 10 mm were eliminated.
Different hydrolysis tests were carried out to define the optimal parameters, such as yield, protein content, and free amino acids in solution, for different ABPs materials to achieve optimal hydrolysis results. Table 2 shows the protein hydrolysates obtained under optimal enzymatic hydrolysis conditions. It must be noted that the final values considered as the results have been obtained from the average values after summing the results of each of the three times the tests were repeated. The content of total and free amino acids shows the potential of the extracted protein hydrolysate to stimulate plant growth by shortcutting the nitrogen cycle of the plants and rapid absorption of the nitrogen present in the amino acids. The Regulation (EU) 2019/1009 establishes the requirement for the total and free amino acid content of biostimulants to produce plant growth stimulation and enhancement.
| Table 2: Composition in protein yield, and total and free amino acids of a hydrolysed product of category 3 wastewater, category 2 PAPs (greaves). | ||
| Hydrolysed product obtained from ABPs | Cat 2 PAPs | Cat 3 wastewater |
| Protein yield (%) | 87.50 ± 2.33 | 97.22 ± 2.45 |
| Total amino acids (% in dry matter) | 70.68 ± 2.27 | 60.72 ± 2.21 |
| Free amino acids (%) in solution | 3.53 ± 0.21 | 5.09 ± 0.26 |
| ABPs: Animal By-Products; Cat: Category | ||
The products obtained show a noticeably high protein recovery, in the range of 87% - 97%, depending on the ABP used. This indicates that the enzymatic hydrolysis developed was efficient for all ABPs. In addition, a high content of total amino acids is observed in the range of 60% - 70%, with the results of the products corresponding to category 2 PAPs being higher than those of category 3 wastewater. This may be of particular interest for its application as a biostimulant.
Amino acids can improve plant performance. For instance, glutamic acid, arginine, alanine, glycine, and proline are the most valuable amino acids for plant growth; glutamic acid, glycine, and aspartic acid improve nutrient uptake; glutamic acid, proline, serine, and valine mitigate the effect of high temperatures; glycine, alanine, glutamic acid, and arginine improve chlorophyll production, and proline, due to its chelating effect, mitigates salinity stress [36-40].
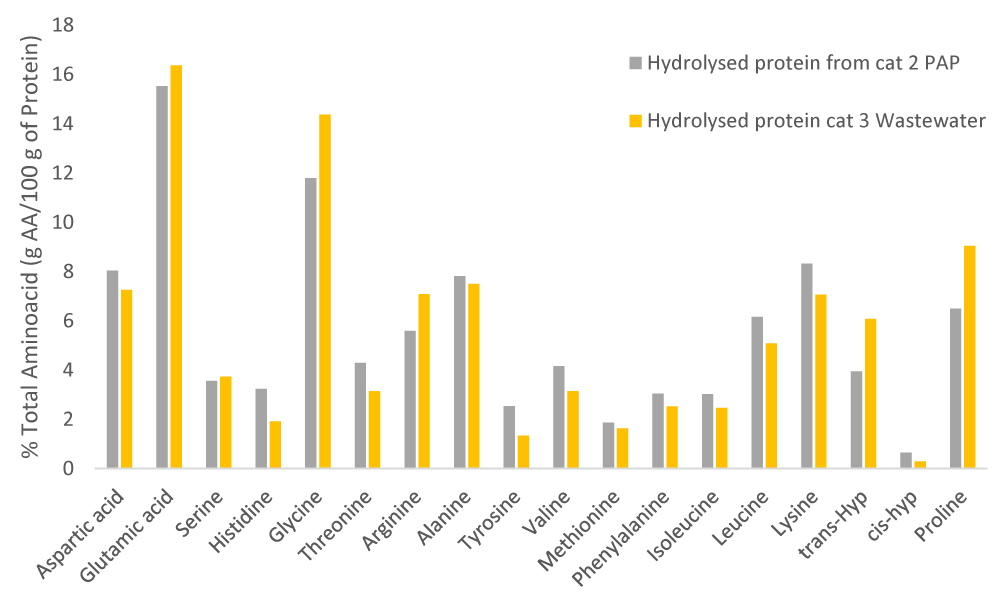
Therefore, in order to evaluate the potential of the recovered protein hydrolysates for application as biostimulants, the amino acids profile of protein hydrolysates was analysed in Figure 2.
Figure 2: Amino acids profile of different protein hydrolysates obtained from category 3 wastewater (yellow) and category 2 PAPs (grey). Source: own elaboration, INESCOP.
The figure above reveals that the glycine value is significantly higher in the hydrolysates from wastewater (14.36%) than in category 2 PAPs (11.79%). Trans-hyp, which represents the collagenic grade of protein, is also considerably higher in hydrolysates from wastewater (6.08%) than in those from PAPs (3.94%), which indicates that the degree of degradation that protein presents for PAPs is considerably higher with respect to wastewater. Proline and glutamic acid follow this same pattern. On the contrary, aspartic acid, alanine, lysine, leucine, and valine present notably higher results for hydrolysates from category 2 PAPs instead for those from category 3 wastewater.
Based on the amino acids profile developed, it can be concluded that the extracted biostimulants will improve nutrient uptake due to the high content of glycine (12% for category 2 PAPs - 14% for category 3 wastewater), glutamic acid (15% for category 2 PAPs -16% for category 3 wastewater) and aspartic acid (7% for category 2 PAPs - 8% for category 3 wastewater). Furthermore, given the proline and glutamic acid values, these biostimulants will mitigate the effect of high temperatures. In this line, it must be noted that the high proline content, due to its chelating effect, will also enable the biostimulants to mitigate salt stress. In addition, serine content (3.73% for category 2 PAPs - 3.56% for category 3 wastewater) and valine (3.14% for category 2 PAPs -4.16% for category 3 wastewater) show the potential of the biostimulants to mitigate the effect of high temperatures [35,36].
Briefly, the amino acids profile shows that both protein hydrolysates are able to improve stress mitigation caused by high temperatures and/or salinity, as well as nutrient uptake. Nevertheless, the protein hydrolysate from category 3 wastewater shows a better composition than category 2 PAPs to improve plant properties.
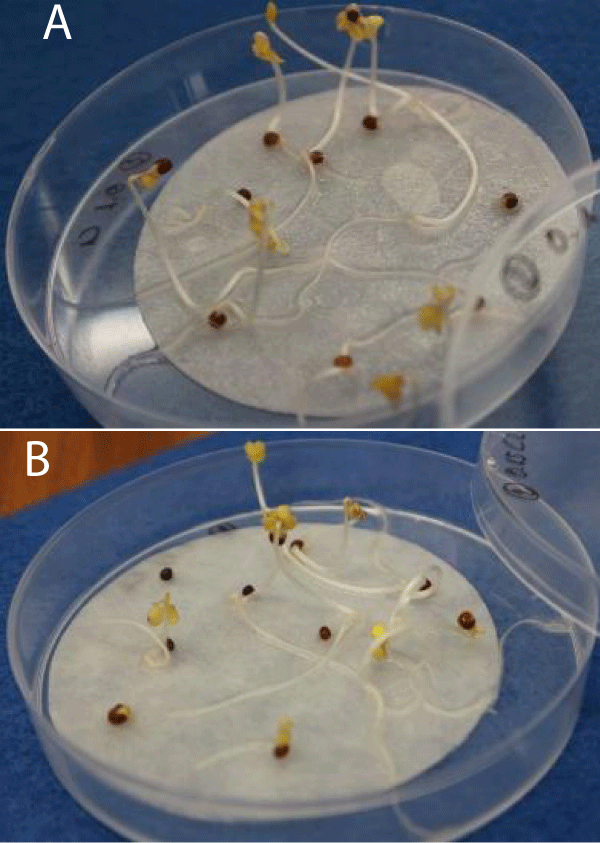
In order to validate the plant growth stimulation potential of the biostimulants developed, their ability to improve the germination growth of different lettuce and Chinese cabbage seeds was evaluated (Figure 3).
Figure 3: Study of the growth of Chinese cabbage and lettuce seeds by dosing different concentrations of biostimulant solution. Chinese cabbage growth using a dosage of 0.1% (A). Lettuce growth using a dosage of 0.15% (B). Source: own elaboration, INESCOP.
Growth improvement during the germination of seeds, due to the biostimulant concentration added, is key to determining the optimum dilution of biostimulant that produces the best growth in Chinese cabbage and lettuce seeds.
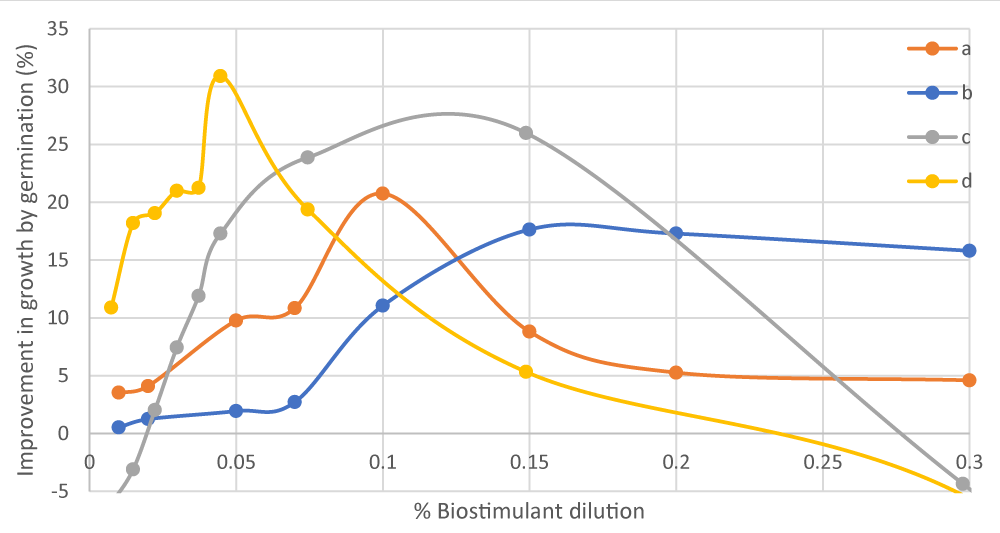
To determine the optimal range of biostimulant dosage, a validation test was carried out through the evaluation of growth by germination in a Petri dish [35]. In this test, two different plant varieties [Chinese cabbage (Brassica rapa pekinensis) and lettuce (Lactuca Sativa)] were watered with 2 ml of different concentrations of protein solution (i.e., biostimulant), 0.01% - 0.3%, and left to grow at 24 ºC for 3 days (Figure 4).
Figure 4: Comparison of the improvement in growth by germination of (a) lettuce from category 3 wastewater hydrolysed protein, (b) Chinese cabbage from category 3 wastewater hydrolysed protein, (c) Chinese cabbage from category 2 PAPs hydrolysed protein, and (d) lettuce from category 2 PAPs hydrolysed protein seeds due to the dosage of the biostimulant obtained. Source: own elaboration, INESCOP.
The results from this validation test revealed that protein hydrolysates from category 2 PAPs provide better improvement in the germination of seeds as well as an optimal concentration, lower than that of protein hydrolysates from category 3 wastewater. This is due to the higher total amino acid content in the biostimulant from category 2 PAPs (70.67%) compared to that from category 3 wastewater (60.72%). This growth effect of the category 2 PAPs biostimulant is also due to its high alanine and arginine content, which improves chlorophyll production, and its serine and valine content mitigates the effect of high-temperature stress.
For protein hydrolysates from category 2 PAPs, 0.05% was the optimal biostimulant dilution for lettuce (Lactuca Sativa) seeds, and for Chinese cabbage (Brassica rapa pekinensis) seeds, optimal results were obtained in the range of 0.1% - 0.15%. On the contrary, hydrolysed protein from category 3 wastewater yielded maximum growth for Chinese cabbage seeds when the biostimulant solution was in the range of 0.12% - 0.15%, and for the lettuce seeds, when the optimal biostimulant dilution was 0.1%.
The dosed concentrations of biostimulants in this study show as biostimulants developed from ABP cat 2 and 3 have the potential to compete with commercial biostimulants.
The results in Figure 4 show the behaviour of Chinese cabbage and lettuce seeds in the presence of total and free amino acids to improve germination speed compared to the test in the absence of amino acids. Figure 4 shows as at adequate concentrations of amino acids, the seeds evaluated have an improvement in germination (17% - 31%) due to the effect of the amino acids in the solution. Finally, it would be interesting to carry out in the future a subsequent study of the amino acid content of the final incubation solution that has been in contact with the seeds in order to evaluate which amino acids have been better absorbed.
The results report considerable differences in the behaviour of the two varieties of lettuce and Chinese cabbage seeds when different concentrations of the biostimulant based on free amino acids were dosed, generating two different profiles. This is due to the fact that lettuce is a plant profoundly sensitive to the pH of the soil where it is grown, with a suitable growth pH interval between 6.7 and 7.4. In addition, an excess of nitrogen can inhibit its growth, therefore, if a higher concentration of (free amino acids) biostimulant than the seed is capable of assimilating is applied, pH will be modified, and growth inhibition will occur. Nevertheless, the needs of Chinese cabbage differ from those of lettuce, and its sensitivity to pH is lower, with adequate growth occurring at pH in the range of 5.8 - 7.9. Thus, the growth interval for Chinese cabbage is remarkably longer.
Two different biostimulants based on free amino acids obtained from category 3 wastewater and category 2 PAPs have been evaluated. A high content of total amino acids has been observed in the range of 60% - 70%. The free amino acid content in the solution corresponds to 3.5% - 5.1%.
The total amino acids distribution corresponding to the high content of glycine, glutamic acid, and aspartic acid shows that the extracted biostimulants will produce improvements in nutrient uptake. The alanine and arginine content will enable these biostimulants to significantly improve chlorophyll production. Furthermore, given the proline and glutamic acid values, the biostimulants will also mitigate the effect of high temperatures. In this line, the high proline content, due to its chelating effect, will allow biostimulants to mitigate salt stress.
Finally, satisfactory results have been obtained, producing an improvement in Chinese cabbage (Brassica rapa pekinensis) and lettuce (Lactuca Sativa) seeds growth between 17% - 31%, with the optimal dilution concentration of the biostimulants in the range of 0.05% - 0.2%, depending on the protein hydrolysate used.
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors thank Granja Otivar, S.L., Footwear Technology Centre, INESCOP, Energygreen Gas Almazan, S.L., TRUMPLER Española, S.A.
Funding: The LIFE by ProtVal project is partially funded by the European Commission through the Life Programme [Project reference: LIFE16/ES/000467].
- European Commission. Integrated Pollution Prevention and Control. Reference Document on Best Available Techniques in the Slaughterhouses and Animal By-products Industries. 2005. https://eippcb.jrc.ec.europa.eu/sites/default/files/2020-01/sa_bref_0505.pdf
- Cheng D, Liu Y, Ngo HH, Guo W, Chang SW, Nguyen DD, Zhang S, Luo G, Bui XT. Sustainable enzymatic technologies in waste animal fat and protein management. J Environ Manage. 2021 Apr 15;284:112040. doi: 10.1016/j.jenvman.2021.112040. Epub 2021 Feb 9. PMID: 33571854.
- Chuck-Hernández C, Ozuna C. Chapter 5 - Protein Isolates From Meat Processing By-Products. In: Galanakis CM, editor. Proteins: Sustainable Source, Processing and Applications. 2019; 131–62. https://www.sciencedirect.com/science/article/pii/B9780128166956000052
- Lapeña D, Vuoristo KS, Kosa G, Horn SJ, Eijsink VGH. Comparative Assessment of Enzymatic Hydrolysis for Valorization of Different Protein-Rich Industrial Byproducts. J Agric Food Chem. 2018 Sep 19;66(37):9738-9749. doi: 10.1021/acs.jafc.8b02444. Epub 2018 Sep 11. PMID: 30142267.
- Morimura S, Nagata H, Uemura Y, Fahmi A, Shigematsu T, Kida K. Development of an effective process for utilization of collagen from livestock and fish waste. Process Biochemistry. 2002 Jul 1; 37(12):1403–12. https://www.sciencedirect.com/science/article/pii/S0032959202000249
- Ohba R, Deguchi T, Kishikawa M, Arsyad F, Morimura S, Kida K. Physiological Functions of Enzymatic Hydrolysates of Collagen or Keratin Contained in Livestock and Fish Waste. Food Science and Technology Research. 2003;9(1):91–3.
- Pagán J, Ibarz A, Falguera V, Benítez R. Enzymatic hydrolysis kinetics and nitrogen recovery in the protein hydrolysate production from pig bones. Journal of Food Engineering. 2013 Dec 1; 119(3):655–9. https://www.sciencedirect.com/science/article/pii/S0260877413003415
- Jayathilakan K, Sultana K, Radhakrishna K, Bawa AS. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: a review. J Food Sci Technol. 2012 Jun; 49(3):278–93. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614052/
- Naveed M, Nadeem F, Mehmood T, Bilal M, Anwar Z, Amjad F. Protease—A Versatile and Ecofriendly Biocatalyst with Multi-Industrial Applications: An Updated Review. Catal Lett. 2021; 151(2):307–23. https://link.springer.com/10.1007/s10562-020-03316-7
- Zhang Y, Olsen K, Grossi A, Otte J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013 Dec 1;141(3):2343-54. doi: 10.1016/j.foodchem.2013.05.058. Epub 2013 May 24. PMID: 23870967.
- Damgaard T, Lametsch R, Otte J. Antioxidant capacity of hydrolyzed animal by-products and relation to amino acid composition and peptide size distribution. J Food Sci Technol. 2015 Oct;52(10):6511-9. doi: 10.1007/s13197-015-1745-z. Epub 2015 Feb 5. PMID: 26396396; PMCID: PMC4573118.
- Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011; 25(8):1813-1827. DOI: 10.1016/j.foodhyd.2011.02.007.
- Gutterres M, daSilva IV. Leather retanning with hydrolyzed protein. Journal of the American Leather Chemists Association. 2010 Jun 1; 105(06):195–202. https://journals.uc.edu/index.php/JALCA/article/view/3267
- Langmaier F, Mokrejs P, Kolomaznik K, Mladek M. Biodegradable packing materials from hydrolysates of collagen waste proteins. Waste Manag. 2008;28(3):549-56. doi: 10.1016/j.wasman.2007.02.003. Epub 2007 Mar 21. PMID: 17376664.
- Akter N, Fatema K, Azad AK, Chakma S. Acid hydrolysis of untanned proteinous wastes from tannery industry in Bangladesh. J Sci Innov Res. 2020 Sep 30; 9(3):83–6. http://www.jsirjournal.com/Vol9_Issue3_01.pdf
- Morimura S, Nagata H, Uemura Y, Fahmi A, Shigematsu T, Kida K. Development of an effective process for utilization of collagen from livestock and fish waste. Process Biochemistry. 2002 Jul 1; 37(12):1403–12. https://www.sciencedirect.com/science/article/pii/S0032959202000249
- Sathish M, Madhan B, Raghava Rao J. Leather solid waste: An eco-benign raw material for leather chemical preparation - A circular economy example. Waste Manag. 2019 Mar 15;87:357-367. doi: 10.1016/j.wasman.2019.02.026. Epub 2019 Feb 15. PMID: 31109536.
- Ammasi R, Victor JS, Chellan R, Chellappa M. Amino Acid Enriched Proteinous Wastes: Recovery and Reuse in Leather Making. Waste Biomass Valor. 2020 Nov 1; 11(11):5793–807. https://doi.org/10.1007/s12649-019-00912-6
- Damrongsakkul S, Ratanathammapan K, Komolpis K, Tanthapanichakoon W. Enzymatic hydrolysis of rawhide using papain and neutrase. Journal of Industrial and Engineering Chemistry. 2008 Mar 1; 14(2):202–6. https://www.sciencedirect.com/science/article/pii/S1226086X07000299
- Hervas F, Celma P, Punti I, Manich A, Cot J, Marsal A, et al. The Enzyme Activity of Trypsen on Sheepskin Trimmings in a Two-Step Collagen Extraction Process. Journal of The American Leather Chemists Association. 2007; https://www.semanticscholar.org/paper/The-Enzyme-Activity-of-Trypsen-on-Sheepskin-in-a-Hervas-Celma/2e7430d448f9213dff47648d2cadf1f4281075d4
- Kanagaraj J, Senthilvelan T, Panda RC, Kavitha S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. Journal of Cleaner Production. 2015 Feb 15; 89:1–17. https://www.sciencedirect.com/science/article/pii/S0959652614011858
- Kumaraguru S, Sastry T, Rose C. Hydrolysis of Tannery Fleshings Using Pancreatic Enzymes: A Biotechnological Tool for Solid Waste Management. Journal of The American Leather Chemists Association. 1998; https://www.semanticscholar.org/paper/Hydrolysis-of-Tannery-Fleshings-Using-Pancreatic-A-Kumaraguru-Sastry/09a0dc35a756c8c2efdf77d6a6d9ddfc73309f89
- Masilamani D, Madhan B, Shanmugam G, Palanivel S, Narayan B. Extraction of collagen from raw trimming wastes of tannery: a waste to wealth approach. Journal of Cleaner Production. 2016 Feb 1; 113:338–44. https://www.sciencedirect.com/science/article/pii/S0959652615018053
- Puhazhselvan P, Pandi A, Sujiritha PB, Antony GS, Jaisankar SN, Ayyadurai N, et al. Recycling of tannery fleshing waste by a two step process for preparation of retanning agent. Process Safety and Environmental Protection. 2022 Jan 1; 157:59–67. https://www.sciencedirect.com/science/article/pii/S0957582021005991
- Selvaraj S, Jeevan V, Rao Jonnalagadda R, Nishad Fathima N. Conversion of tannery solid waste to sound absorbing nanofibrous materials: A road to sustainability. Journal of Cleaner Production. 2019 Mar 10; 213:375–83. https://www.sciencedirect.com/science/article/pii/S095965261833854X
- Sundar JV, Gnanamani A, Muralidharan C, Chandrababu NK, Mandal AB. Recovery and utilization of proteinous wastes of leather making: a review. Rev Environ Sci Biotechnol. 2011 Jun 1; 10(2):151–63. https://doi.org/10.1007/s11157-010-9223-6
- Álvarez C, Lélu P, Lynch SA, Tiwari BK. Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT. 2017; 88:210–216. https://doi.org/10.1016/j.lwt.2017.09.045
- Ansari AJ, Hai FI, Price WE, Drewes JE, Nghiem LD. Forward osmosis as a platform for resource recovery from municipal wastewater - A critical assessment of the literature. Journal of Membrane Science. 2017; 529:195–206. https://doi.org/10.1016/j.memsci.2017.01.054
- Khiari Z, Pietrasik Z, Gaudette NJ, Betti M. Poultry protein isolate prepared using an acid solubilization/precipitation extraction influences the microstructure, the functionality and the consumer acceptability of a processed meat product. Food Structure. 2014; 2:49–60. https://doi.org/10.1016/j.foostr.2014.08.002
- Lo YM, Cao D, Argin-Soysal S, Wang J, Hahm TS. Recovery of protein from poultry processing wastewater using membrane ultrafiltration. Bioresour Technol. 2005 Apr;96(6):687-98. doi: 10.1016/j.biortech.2004.06.026. PMID: 15588771.
- Matak KE, Tahergorabi R, Jaczynski J. A review: Protein isolates recovered by isoelectric solubilization/precipitation processing from muscle food by-products as a component of nutraceutical foods. Food Research International, Innovative food processing technologies: chemical, nutritional and microbiological aspects. 2017; 77:697–703. https://doi.org/10.1016/j.foodres.2015.05.048
- Melchiors MS, Piovesan M, Becegato VR, Becegato VA, Tambourgi EB, Paulino AT. Treatment of wastewater from the dairy industry using electroflocculation and solid whey recovery. Journal of Environmental Management. 2016; 182:574–580. https://doi.org/10.1016/j.jenvman.2016.08.022
- Galanakis CM. Food Waste Recovery: Processing Technologies, Industrial Techniques, and Applications. Academic Press. 2015 https://doi.org/10.1016/C2013-0-16046-1
- Ganju S, Gogate PR. A review on approaches for efficient recovery of whey proteins from dairy industry effluents. Journal of Food Engineering. 2017; 215:84–96. https://doi.org/10.1016/j.jfoodeng.2017.07.021
- Pérez-Aguilar H, Lacruz-Asaro M, Arán-Ais F. Towards a circular bioeconomy: High added value protein recovery and recycling from animal processing by-products. Sustainable Chemistry and Pharmacy. 2022; 28:100667. https://linkinghub.elsevier.com/retrieve/pii/S2352554122000717
- Colla G, Hoagland L, Ruzzi M, Cardarelli M, Bonini P, Canaguier R, Rouphael Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front Plant Sci. 2017 Dec 22;8:2202. doi: 10.3389/fpls.2017.02202. PMID: 29312427; PMCID: PMC5744479.
- Pérez-Aguilar H, Lacruz-Asaro M, Ruzafa-Silvestre C, Arán-Ais F. Protein recovery from wastewater animal processing by-products of rendering plants for biostimulant applications in agriculture. Sustainable Chemistry and Pharmacy. 2023; 32:101009. https://linkinghub.elsevier.com/retrieve/pii/S2352554123000438
- Martín MHJ, Ángel MMM, Aarón SLJ, Israel BG. Protein Hydrolysates as Biostimulants of Plant Growth and Development. In: Ramawat N, Bhardwaj V, editors. Biostimulants: Exploring Sources and Applications. Singapore: Springer Nature Singapore. 2022;141–75. https://link.springer.com/10.1007/978-981-16-7080-0_6
- Pituello C, Ambrosini S, Varanini Z, Pandolfini T, Zamboni A, Povolo C, et al. Animal-Derived Hydrolyzed Protein and Its Biostimulant Effects. In: Ramawat N, Bhardwaj V, editors. Biostimulants: Exploring Sources and Applications. Singapore: Springer Nature Singapore; 2022. p. 107–40. https://link.springer.com/10.1007/978-981-16-7080-0_5
- Povolo C, Avolio R, Doria E, Marra A, Neresini M. Development and validation of an analytical method to ensure quality requirements of hydrolysed proteins intended for agricultural use as biostimulants. Talanta Open. 2022 Aug 1; 5:100082. https://www.sciencedirect.com/science/article/pii/S2666831922000017