More Information
Submitted: June 16, 2022 | Approved: September 19, 2022 | Published: September 20, 2022
How to cite this article: Mekonnen TB. An overview of the developments of nanotechnology and heterogeneous photocatalysis in the presence of metal nanoparticles. J Plant Sci Phytopathol. 2022; 6: 103-114.
DOI: 10.29328/journal.jpsp.1001083
Copyright License: © 2022 Mekonnen TB. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nanotechnology; Heterostructure; Photocatalyst; Doping; Coupling; Photocatalysis
An overview of the developments of nanotechnology and heterogeneous photocatalysis in the presence of metal nanoparticles
Tigabu Bekele Mekonnen*
Department of Chemistry, Mekdela Amba University, Tuluawuliya, Ethiopia
*Address for Correspondence: Tigabu Bekele Mekonnen, Department of Chemistry, Mekdela Amba University, Tuluawuliya, Ethiopia, Email: [email protected]
In general, nanotechnology can be understood as a technology of design, fabrication and applications of nanostructures and nanomaterials, as well as a fundamental understanding of the physical properties and phenomena of nanomaterials and nanostructures. In recent years the development of industries like textile, leather, paint, food, plastics, and cosmetics is enlarged and these industries are connected with the discarding of a vast number of organic pollutants which are harmful to microbes, aquatic systems, and human health by influencing the different parameters. So the fabrication of those nanomaterials (coupled or doped) to form heterojunctions provides an effective way to better harvest solar energy and facilitate charge separation and transfer, thus enhancing the photocatalytic activity and stability. We expect this review to provide a guideline for readers to gain a clear picture of the fabrication and application of different types of heterostructured photocatalysts. In this review, starting from the photocatalytic reaction mechanism and the preparation of the photocatalyst, we review the classification of current photocatalysts, preparation methods, a factor that affects photocatalytic reaction, characterization of photocatalysts, and the methods for improving photocatalytic performance. This review also aims to provide basic and comprehensive information on the industrialization of photocatalysis technology.
Due to the development of various industries in modern society, water pollution has become one of the most serious environmental problems. Polluted water not only affects the ecological system, causing damage to the living in water and those relying on the aquatic animals and plants but also threatens human beings. One of the most common contaminants in wastewater is organic substances, most of which are very stable in the natural environment [1]. Hence, they can aggregate in wastewater and are therefore harmful to the aquatic ecosystem. Unfortunately, many industries are producing organic pollutants containing wastewater, which aggravates the water pollution problems [2]. Dye wastes represent one of the most problematic groups of pollutants because they can be easily identified by the human eye and are not easily biodegradable. Generally, dyes are very stable to light and oxidation due to the complex aromatic molecular structures, but this causes damage to the environment and dramatically threatens human health [3-5]. Therefore, how to treat wastewater, especially removing organic pollutants in wastewater is one of the most crucial problems for sustainable development [6].
Various conventional treatment methods for dye removal from wastewater include physical, chemical, and biological processes such as anaerobic treatment, trickling filters, flotation, chemical coagulation, electrochemical coagulation, and membrane separation, which have been studied so far [7]. However, the main disadvantages of these methods include the production of toxic sludge, high operational cost, technical limitations, lack of effective color reduction, and sensitivity to a variable wastewater input. It is also a problem because these dye compounds in wastewater ordinarily contain one or several benzene rings and cannot be decomposed easily in chemical and biological processes. Moreover, most of the dyes are found to be resistant to the normal treatment process.
The use of solar energy and semiconductor catalysts for photocatalytic degradation of organic dyes in water has been intensively investigated as an emerging renewable technology [8-11]. Photocatalysis is one of the Advanced Oxidation Processes (AOP) considered an efficient, stable, and environmentally friendly method in the field of environmental pollution control [12]. It is known that the photocatalysis process can proceed under UV light or/and visible light irradiation. Under light irradiation, active species are generated on the catalyst surface to react with the organic contaminants in the wastewater. During this process, the organic contaminants can be partially degraded to smaller molecules or even completely mineralized to CO2 and H2O [13]. Since visible light occupies much higher spectral power within the solar spectrum than UV light, visible light prompted photocatalysis is more advantageous for its efficient utilization of solar irradiation [14]. Therefore, developing a photocatalyst that can efficaciously degrade organic contaminants under visible-light irradiation is a highly promising direction in the application of wastewater treatment.
Several semiconductor photocatalysts are being used for the treatment of wastewater pollutants. Among them, TiO2, ZnO, CeO2, CdS, Ag3PO4, etc... has been reported as a new UV or visible-light-driven photocatalyst for the oxidation of water and photodecomposition of organic compounds [15-18]. To further improve the photocatalytic performance and stability of every single photocatalyst, combining it with a metal or other semiconductor to form hybrid materials is an effective way to promote the separation of photogenerated charge carriers and thus enhance the photocatalytic activity and stability.
Band gap modifications can be carried out by using several approaches such as doping of metal and non-metal elements, metal-metal co-doping, nonmetal-nonmetal co-doping, metal-nonmetal co-doping [19], tri-doping, dye sensitization, deposition of noble metals, and making composite photocatalyst by forming heterojunctions [20-24]. Among the various techniques for the enhancement of visible light effective photocatalysis, composite photocatalysts have drawn more attention owing to their significant increase in photocatalytic activity. The formation of heterojunction between two semiconductors allows the interaction of the band structure which effectively prevents the electron-hole recombination thereby enhancing the photocatalytic activity [25]. Thus, in order to control the wide band gap of the ZnO nanoparticles, combining two or more semiconductor nanoparticles with different band gap energy has been used, and combining different semiconductor oxides can reduce the band gap, extending the absorbance range to the visible light region leading to electron-hole pair separation under irradiation and consequently, achieving a high photocatalytic activity [24,26].
Nanostructured semiconductors
Applying the concept of nanotechnology to heterogeneous catalysis helps us to understand more accurately the transformations occurring on the catalyst´s surface at a molecular level. The synthesis of materials with nanometric dimensions will facilitate a better understanding of the reaction mechanisms as well as to design of novel useful catalytic systems. Nanostructured photocatalysts offer a large surface-to-volume ratio allowing higher adsorption of the target molecules. Intensive research over the past decade on its implementation in the purification of drinking water can be found in the literature [27,28]. Photocatalysis, using nanostructures of metal oxide semiconductors like zinc oxide (ZnO), titania (TiO2), and ceria (CeO2) can be an attractive way of water purification as it is capable of removing chemical as well as biological contaminants [29,30]. A good photocatalyst should absorb light efficiently preferably in the visible or near UV part of the electromagnetic spectrum. Sufficient electron vacant states need to be present to inhibit the recombination of electron-hole pairs upon light exposure.
Since the discovery of heterogeneous photocatalysis [31], numerous efforts have been carried out to understand the mechanism of the reaction and how to select the main variables that affect its efficiency. Several authors have associated the efficiency of a semiconductor photocatalyst with electronic, structural, and morphological properties of the material such as band-gap energy (Eg), crystalline structure, surface area, and morphology. The possibility of controlling these variables is very interesting from a technological point of view because the development of materials with high photocatalytic activity allows their application to environmental fields such as wastewater treatment [32]. Thus, the development of nanostructured semiconductors for catalytic applications provides materials with a high value in their surface/volume ratio [33].
Effect of size
In particular, due to the small size of the particles, the quantum effect affects directly some physical properties of the material such as optical and electronic properties [34]. Such a situation has opened numerous fields of technological application of these materials, which include the development of new generations of solar cells, production of hydrogen, and elimination of pollutants from wastewater. From a point of view of photocatalytic applications, the small size of particles of nanostructured semiconductors plays an important role in the activity of photocatalysts. As is well known, the critical step in heterogeneous photocatalysis is the undesirable recombination of pair electron-hole before they reach the catalyst surface. In this sense, the small size of prepared particles reduces the pathway from the site of generation of the pair electron-hole to the catalyst surface.
Effect of composition
In heterogeneous catalysis, the reacting molecules adsorb on the catalytically active solid surface. Chemical bonds are broken and formed on the surface and eventually, the products are released back into the liquid or gas phase. Many of the heterogeneous catalysts used in industry today consist of small particles of catalytically active material, typically with a diameter of 1-10 nm, anchored on porous support [35]. The use of nanoparticles results in a large contact area between the active material of the catalyst and the surrounding gas or liquid phase. This ensures that the catalytic material is used effectively. One of the interesting scientific and technological challenges associated with the use of nanoparticles as catalysts is the understanding of how the composition and atomic-scale structure of nanoparticles produce the best catalytic activity. The second challenge is to synthesize these particles with maximum control over the composition and structure. Modern nanotechnology methods clearly offer great potential for future developments in both the characterization and synthesis of heterogeneous catalysts based on supported nanoparticles.
Metallic nanoparticles finely dispersed over oxide supports have found use as heterogeneous catalysts in many industries including chemical manufacturing, energy-related applications, and environmental remediation. The compositional and structural complexity of such nanosized systems offers many degrees of freedom for tuning their catalytic properties. For example, a higher surface area is desirable in a heterogeneous catalyst, in order to get a higher concentration of active sites where a molecule of pollutant can be anchored to undergo its decomposition by the interchange of carriers coming from excitation of semiconductor. To reach this condition, TiO2 nanostructured with zero dimensionality due to the shape of the sphere of its particle can be developed. The application of nanostructured TiO2 for wastewater treatment usually includes the elimination of organic compounds with large and complex molecular structures. For this reason, steric effects in the adsorption of molecules of organic pollutants over the surface of the photocatalyst should be taken into account. In this sense, two-dimensional nanosheets have smooth surfaces which leads to a high adhesion of organic molecules [36].
Approaches to make photocatalysis visible active
There are different methods for making zinc oxide active in visible light thereby increasing its photocatalytic activity for various applications. Some of the methods are discussed as follows:
Doping
Metallic doping: The use of semiconductors doped with a transition metal as photocatalysts has been extended in order to improve their physical properties, in particular, to induce a narrow energy band gap in its electronic structure. This situation can lead to producing materials with better photocatalytic activity than the well-known TiO2, in particular, to take advantage of the energy that comes from the visible region of the solar spectrum. However, the poor photo efficiency in the process of separation of charges under light excitation limits its future application. To solve this problem, the doped of metal transition oxides with a great variety of transition metal ions represents an interesting expectative, in particular, to enhance the photocatalytic activities of the oxide. For example, the semiconductor oxide ZnO with Wurtzite structure is one of the most studied photocatalysts after TiO2 due to its high electrochemical stability and nontoxicity. Several works revealed this oxide as a promising candidate to solve environmental pollution problems related to water purification. Thus, reducing the energy band gap of the oxide by doping. Improvement in the activity of Ce-doped ZnO to the photocatalytic degradation of methylene blue in solution was attributed to the presence of a higher concentration of oxygen defects on the surfaces of crystalline-doped material [37,38].
Nonmetal-doping: Doping with carbon, nitrogen, sulfur, and other non-metallic elements have been reported to introduce visible light absorption on zinc dioxide. Substitutional N-doping generates a new band close to the VB of ZnO with which electrons from the valence band of ZnO make a two-step transition to the conduction band using visible light. As result, photocatalytic activities of ZnO enhanced when compared with pure ZnO [39].
Coupling
To increase light utilization efficiency, a variety of approaches have been developed to engineer the oxide structures and properties, such as doping with metal and nonmetal elements, combination with other semiconductor materials, and sensitization by organ metallic dye molecules. In these, the formation of a stepwise band gap structure in the composites with other semiconductor materials has been found to lead to superior photocatalytic performance because the resulting catalysts not only extend light absorption to the visible region but also exhibit a reduced recombination rate of photogenerated electron-hole pairs. The association of two or more nanocrystalline semiconductors allows the design of semiconductors heterostructures that are potentially useful in photocatalysis, water splitting, microelectronics, and others [40,41].
Doping with coupling
However, metals and nonmetals doping enhance visible light utilization of semiconductors, in photocatalytic degradation of organic pollutants in wastewater treatment techniques. Doping with coupling has a tremendous effect on photocatalytic activities than only doping in heterogeneous photocatalysis. Thus, photocatalysts such as Fe codoped Sn-TiO2 and CuO coupled Sn-TiO2 were successfully developed and used in the photocatalytic degradation of methyl orange and phenol derivatives. The iron oxide coupled and doped Titania has excellent long-term stability and could perform the renewable photocatalytic activity. Results indicate that this material will be a promising visible light-active photocatalyst for the treatment of wastewater.
Amongst the available strategies, the formation of heterojunctions has attracted great attention due to the effectiveness of separating photogenerated charges as well as the improvement of photocatalytic activity [19]. For example, the formation of Z-scheme junctions enables the use of n-type and p-type semiconductors for overall water splitting, while they can only achieve water oxidation or water reduction half-reaction when used alone. Even though there are several review papers reporting heterojunctions in recent years, due to the dynamic development of this research field, a comprehensive review focused on the rational design of visible light responsive heterojunctions is still necessary to provide readers with a better understanding of the state-of-the-art progress in this dynamic research field [42,43].
Heterojunction photocatalysts
To improve the photocatalytic activities of a single photocatalyst, a good strategy is the coupling two semiconductors to form composite photocatalysts, because it has been reported that if two semiconductors are properly integrated into one system, this system can be expected to achieve high photocatalytic activity [44].
Visible light-responsive photocatalysts are fundamentally important for efficient photocatalysis. The design and synthesis of visible light responsive heterojunctions have been focused on the improvement of light harvesting, suppression of photogenerated charge recombination, and enhancement of photocatalytic activity [19,43]. According to the materials involved, heterojunctions can be primarily divided into five types: semiconductor/semiconductor (S/S) junctions, semiconductor/cocatalyst (S/C) junctions, semiconductor/metal (S/M) junctions, semiconductor/ non-metal (S/N) junctions, and surface heterojunctions. From those heterojunctions, S/S junctions (p-n and n-n) are discussed in detail in this review.
S/S junctions: Based on the band arrangements of semiconductors, S/S junctions can be classified into straddling gap (type I) junctions, staggered gap (type II) junctions, and broken gap (type III) junctions [45] Amongst these three junctions, type II junctions are the most effective structure for the separation of photogenerated electrons and holes, which has been widely studied in recent years [46]. According to the mechanism of charge transfer and separation between the contacted semiconductors, three efficient junctions including p-n junctions, n-n junctions and Z-scheme junctions have been explored, which will be discussed in detail in this review section.
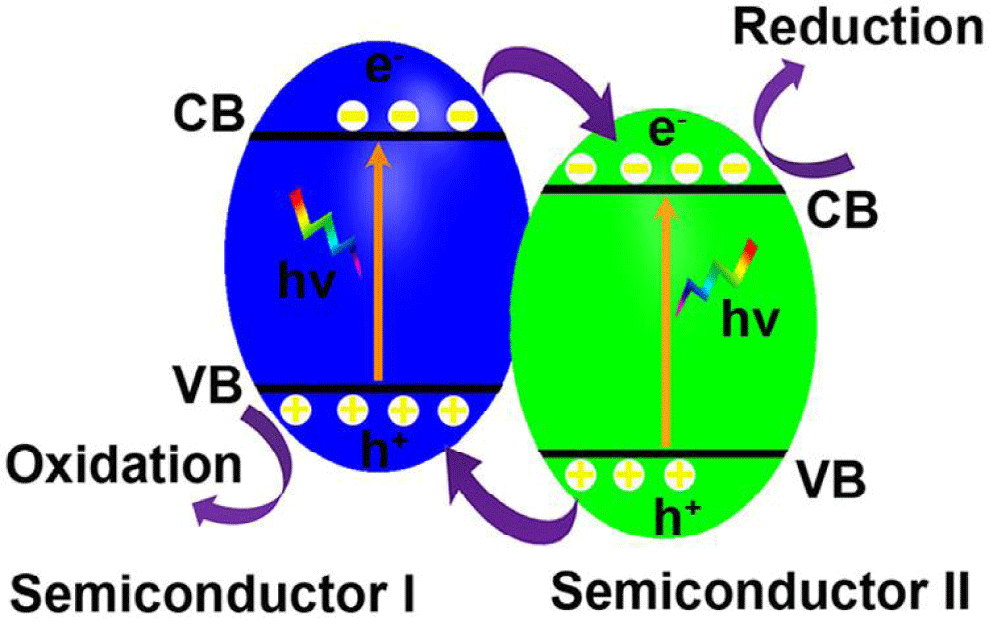
p-n junctions: The fabrication of p-n junctions composed of p-type and n-type semiconductors is effective to separate the photogenerated electrons and holes due to the formation of built-in electrical potential in the interfaces caused by the equilibration of Fermi levels, which transfers the photogenerated electrons from the CB of the p-type semiconductor to that of the n-type semiconductor while driving the holes from the VB of the n-type semiconductor traveling to that of the p-type semiconductor (Figure 1). Combining narrow bandgap p-type semiconductors with large bandgaps n-type semiconductors such as TiO2 and ZnO to form p-n junctions not only shifts the light absorption edge from the UV region to the visible light region but also effectively reduces the recombination of photogenerated holes and electrons. In recent years, visible light responsive p-n junctions such as Ag3PO4/CeO2 [47,48], Cu2O/TiO2 [49], Ag3PO4/TiO2 [50], CuO/ZnO [51] and Cu2O/ZnO [52] are synthesized.
Figure 1: Schematic illustration of p-n junction band alignments and the correspondingly possible photoexcited electron-hole pair separation and transfer between them contacted semiconductors.
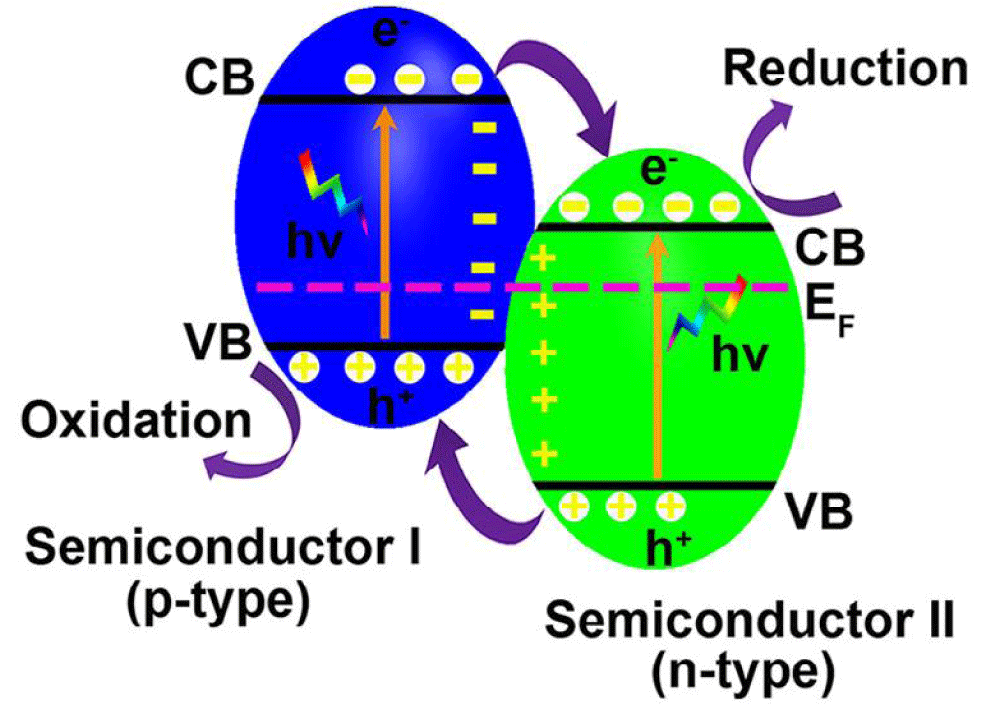
n-n junctions: In n-n junctions, the positions of CB and VB of n-type semiconductor I am both more negative than that of n-type semiconductor II. Different chemical potentials between the two semiconductors cause band bending at the interface, forming a built-in-field, which effectively drives the photogenerated electrons to move from the CB of semiconductor I to that of semiconductor II and holes from the VB of semiconductor II to that of semiconductor I (Figure 2) [19,53].
Figure 2: Schematic illustration of n-n junction band alignments, possible photoexcited electron-hole pair separation, and transfer between the contacted semiconductors.
Generally, narrow bandgap n-type semiconductors can help large bandgap semiconductors such as CeO2, TiO2, ZnO, and SnO2 to utilize visible light by forming n-n junctions. CdS/CeO2 junctions prepared via a simple precipitation method showed high activity for photocatalytic hydrogen production under visible light illumination for 120 h [54]. In addition, other narrow bandgap/large bandgap visible light responsive n-n junctions such as CeO2/ZnO [55], CeO2/TiO2 [56], AgBr/TiO2 [57], C3N4/TiO2 [58] and Fe2O3/ZnO [59] also showed enhanced photocatalytic activity under visible light illumination. The formation of n-n junctions promotes the separation of photogenerated electrons and holes in narrow bandgap semiconductors, thus dramatically improving the photocatalytic activity when compared to the bare narrow bandgap semiconductor itself.
Multiple n-n junctions are proposed to further improve photocatalytic performance due to the enlargement of light absorption and better charge separation. For example, g-C3N4/CeO2/ZnO [60], CeO2-ZnO/TiO2 [61] and TiO2/CdS/CdSe [62] junctions exhibited remarkable enhancement of photocatalytic activity.
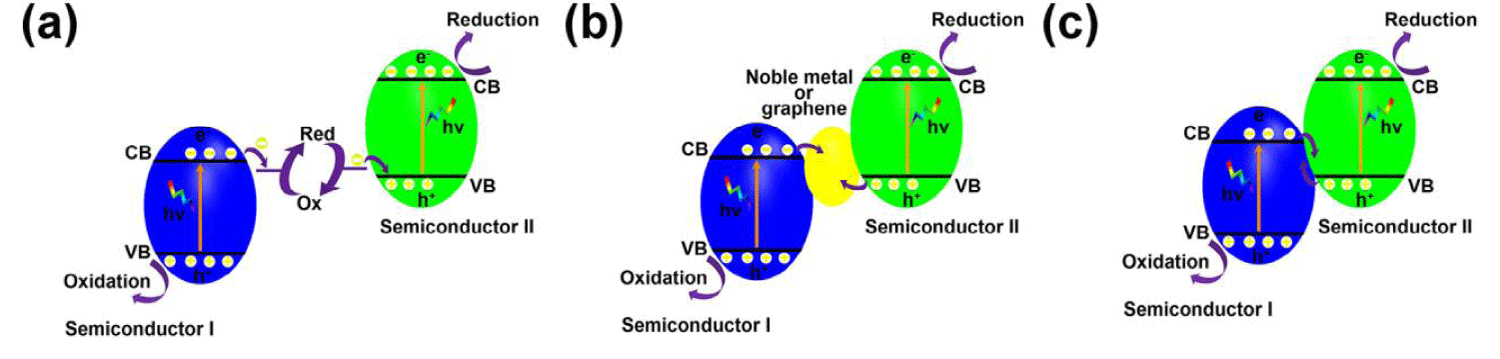
Z-Scheme junctions: Generally, Z-scheme junctions are composed of semiconductor I with CB more negative than the hydrogen generation potential, semiconductor II with VB more positive than the oxygen generation potential, and a liquid-state or solid-state electron mediator that can recombine holes from VB of semiconductor II and electrons from CB of semiconductor I, resulting in overall water splitting (Figure 3) [63]. Unlike p-n junctions and n-n junctions mentioned above, Z-scheme junctions are able to keep photogenerated electrons and holes in strongly redox states, which is promising for water splitting [64], CO2 reduction [65], and pollutant degradation [66].
Figure 3: Schematic illustration of Z-scheme junction band alignments, possible photoexcited electron-hole pair separation, and transfer between the contacted semiconductors: (a) with liquid-state electron mediator, (b) with solid-state electron mediator, (c) without electron mediator.
Redox couples such as IO3-/I- [67], I3-/I- [68], Fe3+/Fe2+ [69] and NO3-/NO2- [70] are widely used as liquid-state electron mediators for Z-scheme junctions, which can achieve overall water splitting. Pt/SrTiO3: Rh/(BiVO4, Bi2MoO6 or WO3)/Fe3+/2+ [71], Ru/SrTiO3: Rh/BiVO4/[Co-(bpy)3]3+/2+ [72], CdS/Au/TiO1.96C0.04 [73], Ru/(SrTiO3: La/Rh)/Ir/ CoOx/Ta3N5 [19], H2WO4·H2O/Ag/AgCl [74], Ag3PO4/Ag/AgI [75] are some of Z-scheme junctions. Even though redox couples-based Z-scheme junctions can achieve overall water splitting, these systems are limited to liquid phase photocatalytic reactions only, which hinders their applications in other fields such as photocatalytic pollutant degradation in solutions [63]. Moreover, the redox couples all inevitably absorb light and their long-term stability in a wide range of pH is challenging. Therefore, all solid-state Z-scheme systems attracted increasing attention in the past decade. Noble metals such as Au, Ir, Pt, and Ag can be used as solid-state electron mediators.
Principles of photocatalytic degradation
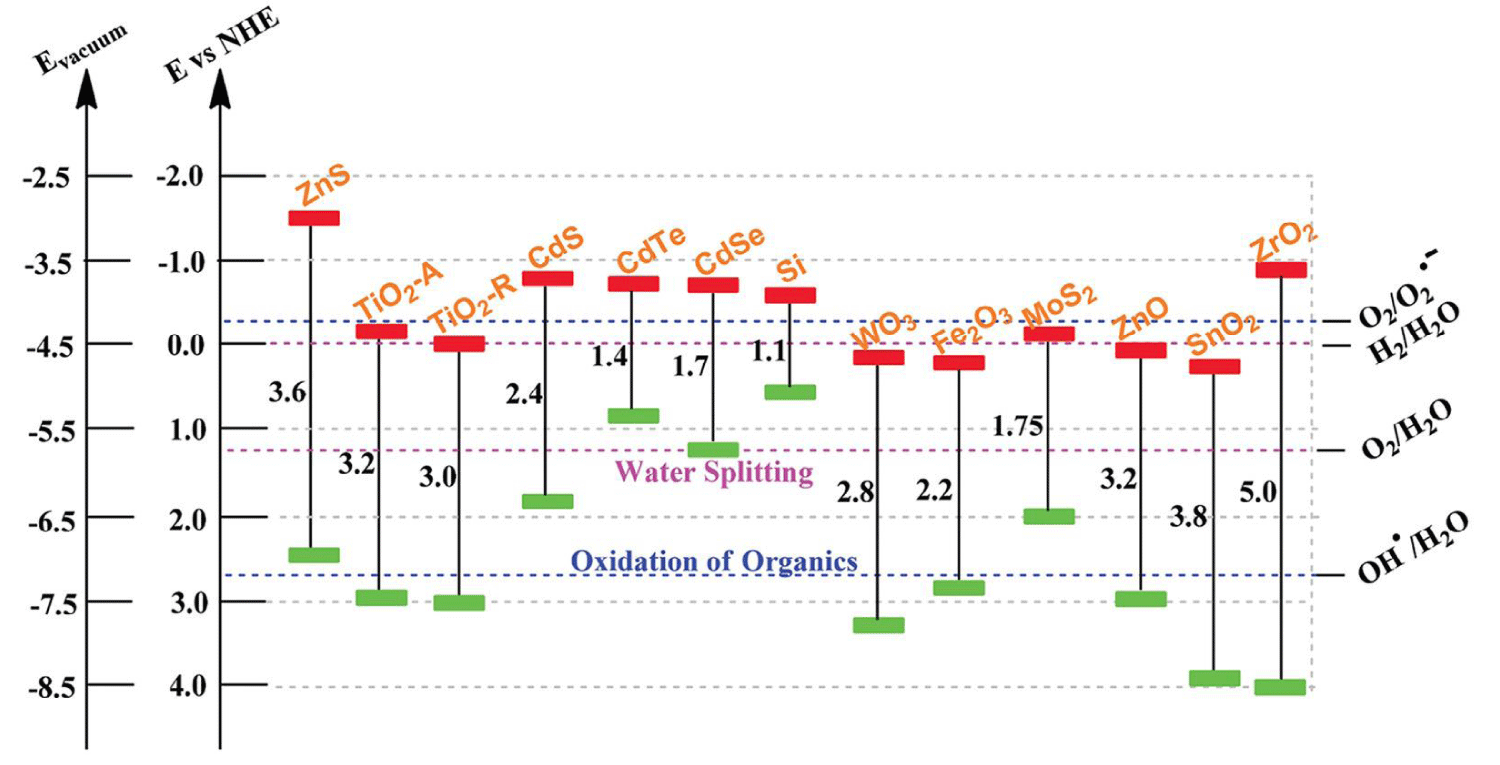
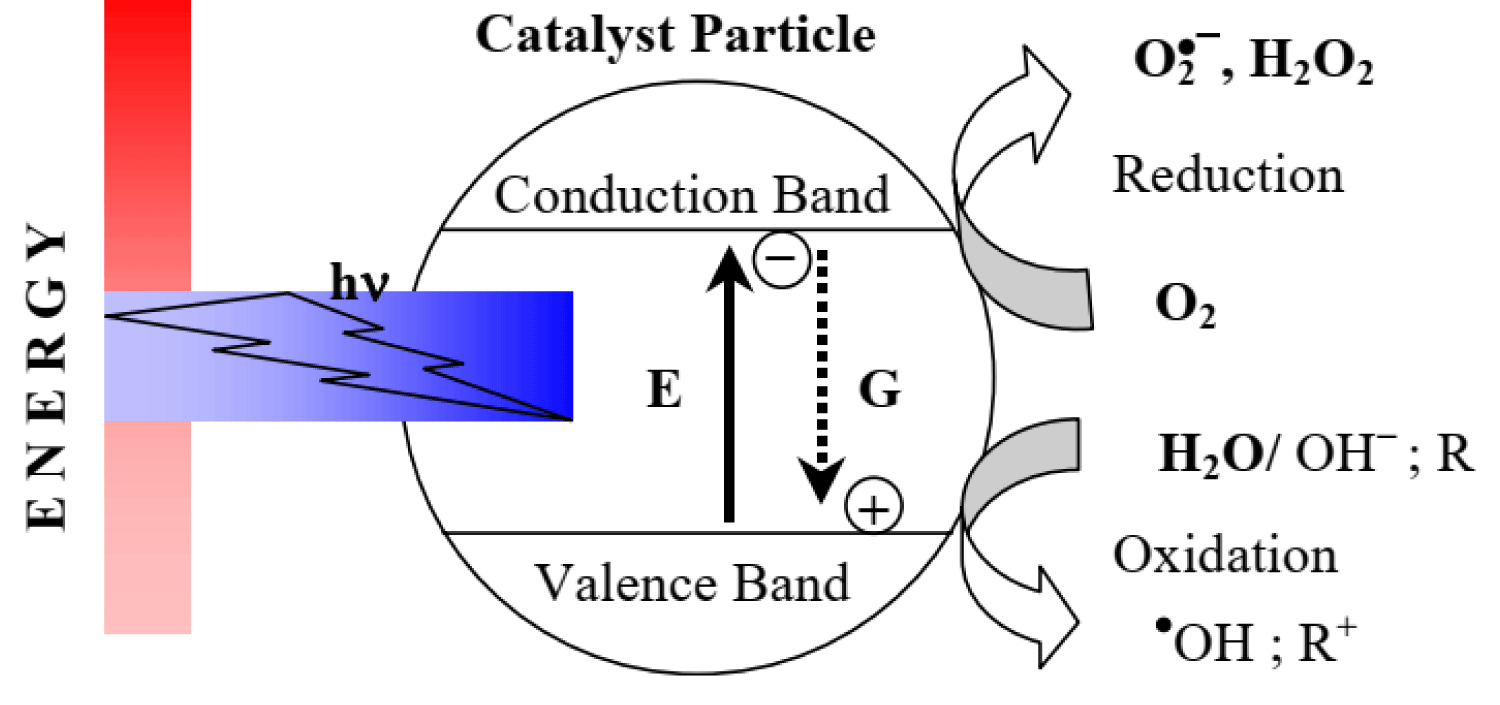
In the photocatalytic degradation process, organic pollutants are destroyed in the presence of a semiconductor photocatalyst, an energetic light source, and an oxidizing agent such as oxygen or air. Only photons with energies equal to, or greater than the band gap energy can result in the excitation of valence band (VB) electrons which then promote the possible reactions with organic pollutants. The absorptions of a photon with energies lower than the band gap energy usually cause dissipation in the form of heat. The illumination of the photocatalyst with sufficient energy leads to the formation of the positive hole (h+) in the valence band and an electron (e-) in the conduction band (CB). The positive hole oxidizes either the pollutants directly or water to produce hydroxyl radical: •HO, whereas the electron in the conduction band reduces the oxygen adsorbed on the photocatalyst [76]. For example, only semiconductors that possess a CB that is more negative than 0 V vs. normal hydrogen electrode (NHE) and a VB that is more positive than 1.23 V vs. NHE are able to split water into hydrogen and oxygen, as shown in Figure 4 [77]. In this regard, very few semiconductors can be used for photocatalytic water splitting. On the other hand, the photogenerated electrons and holes are in energetically unstable states and thus tend to recombine, which restricts photoconversion efficiency. Therefore, in addition to developing promising visible light-responsive semiconductors, strategies are still needed to achieve high efficiency for commercial possibility.
Figure 4: Bandgap energy, VB and CB positions of several common semiconductors on a potential scale (V) vs. NHE/vacuum.
The possible mechanism of degradation of the organic compound using an irradiated photocatalyst (ZnO) is described below:
1. Absorption of efficient photons (hv > Eg).
Photocatalyst + hv→ h+ (VB) + e- (CB) (1)
2. Oxygen ion sorption (first step of oxygen reduction; oxidation state of oxygen changes from 0 to -1/2).
(O2) ads + e (CB) – → O2- (2)
3. The -OH groups combine by photo holes which produce -OH radicals.
(H2O = H+ + -OH) ads + h+VB → •OH + H+ (3)
4. The O2•- combined with protons produces HO2•
2•- + H+ → HO2• (4)
5. Oxidation of the organic reactant via successive attacks by •OH radicals.
Organic (R) + •OH + O2 → CO2 + H2O + other products (5)
The energy required for electron excitation depends on the particular characteristics of the semiconductors. The λmax required to promote an electron depends upon the band gap energy, (Eg) of the photocatalyst and is given by the following equation. Eg = 1240/λmax Semiconductors can act as sensitizers for light-reduced redox processes due to their electronic structure, which is characterized by a filled valence band and an empty conduction band. When a photon matches or exceeds the Eg of the semiconductor, an electron (e-CB) is promoted from the valence band into the conduction band leaving a hole behind. Adsorbed oxygen at grain surface is an electron capture, which can restrain the combination of electron and hole. The nano-scale catalysts have higher photocatalytic activity than normal material [78]. There are two reasons for this:
1. Quantum size effect: When the particle diameter becomes less than a certain critical value, the valence band and conduction band change into discrete levels, and the energy gap becomes broader, which means, the valence band electric potential becomes more positive and the conduction electric potential becomes more negative. Then the oxidation-reduction capability of the electron and hole is enhanced and the photocatalytic activity of the nano semiconductor is improved.
2. Specific surface area: At the higher specific area of particles there are more atoms on the surface; it improves the adsorption capability of semiconductors for organic pollutants: the time spent by electrons and holes to get to the surface of the particle decreases. The smaller the particle diameter, the shorter is time spent by carriers diffusing from inside to the surface. It can get higher separated efficiency and lower probability of electron-hole recombination. Therefore, the nano semiconductor has more photocatalytic efficiency than the common semiconductor. In general photocatalysis degradation of organic effluents is described briefly as follows Figure 5.
Figure 5: Schematic diagram of photocatalytic degradation of organic effluent in wastewater.
Factors affecting photocatalytic process
There are a number of factors that govern the photodegradation of a dye. Photocatalytic reactions are extremely complex processes involving many participants namely water, organic substrate, catalyst, light, and oxygen. Therefore, it is obvious that operational parameters related to each of these agents may affect the efficiency of the photocatalytic process. Photocatalytic reaction rates are known to be affected by several operation conditions including pH of the medium, catalyst loading, substrate concentration, intensity of light, temperature, and oxygen pressure. Also, the physical and chemical intrinsic properties of the photocatalyst may affect its photo efficiency [79].
Effect of photocatalyst load
The initial rates of reaction are directly proportional to the mass (m) of the catalyst. However, above a certain value of m, the reaction rate levels off and becomes independent of mass [80]. The increase in the efficiency seems to be due to the increase in the total surface area (active sites) available for the photocatalytic reaction as the dosage of the photocatalyst increased. However, when the catalyst was overdosed, the number of active sites on the catalyst surface may become almost constant because of the decreased light penetration via the shielding effect of the suspended particles and the loss in the surface area caused by agglomeration [81].
Effect of initial dye concentration
As the concentration of the model pollutant increases, more molecules get adsorbed on the photocatalyst surface, the substrate concentration can influence the extent of adsorption and rate of reaction at the surface of the photocatalyst. It will be an important parameter for optimization between high degradation rate and efficiency. Mahalakshmi and his coworker found the optimum concentration for the dye under investigation and the rate increases up to this point but above this concentration, the rate decreases due to insufficient quantity of •OH radicals, as the formation of •OH radicals is constant for a given amount of the catalyst [82]. Similarly, Zhu and his coworker reported that the photo generation of holes or •OH radicals on the catalyst surface is reduced since the active sites are covered by dye ions [83]. Another possible cause is the radiation screening effect at a high dye concentration since a significant amount of radiation may be absorbed by the dye molecules rather than the photocatalyst particles and then reduces the efficiency of the catalytic reaction [84].
Effect of surface area of a photocatalyst
The surface area of a solid catalyst is directly related to the concentration of active sites for adsorption and reaction. A large surface area can be a determining factor in certain photodegradation reactions since the adsorption of large amounts of substrate and oxygen promote the reaction rate. However, powders with high surface areas are usually associated with large amounts of crystal lattice defects, facilitating the recombination of the photo-generated electron/hole pairs, and leading to poor photocatalytic activity [85]. It has been reported that the photocatalytic activity of amorphous Titania is negligible indicating that crystallinity is an important requirement. Therefore, a balance between surface area and crystallinity must be found in order to obtain the maximum photo activity [86].
Effect of initial pH of dye solution
The solution pH is an important variable in aqueous phase photocatalytic reactions. The pH of a solution influences the adsorption and dissociation of the substrate, catalyst surface charge, oxidation potential of the valence band, and other physicochemical properties of the system [80]. In accordance with Nerst’s law, varying the solution pH would shift the energy of the valence and conduction band edges [13]. This results in the conduction band electron becoming more effective and the valence band holes less effective at higher pH. The pH affects significantly not only photocatalyst activity but also changes pollutant structure. For example, phenol can be charged positively or negatively under different pH ranges. The interaction and affinity between both photocatalyst and phenol will be varied with the solution pH. So, the pH of the aqueous solution is a key factor for photocatalytic reaction and can affect the adsorption of pollutants on the photocatalyst surface, an important step for photo-oxidation to take place [87]. The degradation rate of phenol decreased with the increase in pH. Moreover, a low degradation rate at higher pH is attributed to the fact that when the concentration of OH- ion is higher in the solution, it prevents the penetration of UV light to reach the catalyst surface. Furthermore, high pH favors the formation of carbonate ions which are effective scavengers of OH- ions and can reduce the degradation rate [84].
Synthesis methods of photocatalysts
Hydro - and solvo-thermal synthesis: Hydrothermal synthesis involves water as the solvent whereas solvothermal refers to the use of organic solvents. The choice of solvent is based on its ability to dissolve the organic linker. In both cases, the reactions take place inside a Teflon-lined stainless steel autoclave. Since it is a closed system, an autogenous pressure will be built up. The reaction mixture is normally heated at temperatures ranging from 80 to 220 oC, over a time of several hours to several days. Compared with microwave, electrochemical and mechanochemical techniques; the use of autoclaves is a slow method [88,89].
Co-precipitation method: A facile and convenient method to prepare nanoparticles is the chemical co-precipitation technique. Two or more soluble salt solutions are mixed in a definite ratio and co-precipitated with a base solution under an inert atmosphere. Solutions of two or more water-soluble salts of metals are dissolved in water, mixed, and co-precipitated with alkali very slowly. At a simple level, precursors such as nitrates and carbonates can be used as starting materials instead of oxides: they decompose to the oxides on heating at relatively low temperatures, losing gaseous species, and leaving behind fine, more reactive powders [90]. An even more intimate mixture of starting materials can be made by the co-precipitation of solids. A stoichiometric mixture of soluble salts of the metal ions is dissolved and then precipitated as hydroxides, citrates, oxalates, or formats. This mixture is filtered, washed, dried, and then heated to give the final product. An increase in the mixing rate decreased the size of the nano photocatalyst. It is a simple method that takes place at a lower temperature than hydrothermal or thermal decomposition. The solvent used is environmentally friendly and the yield is high [91].
Microwave synthesis
Microwave synthesis has lately been shown to have a big impact, is more environmentally friendly, and less energy is required compared to conventional heating for the synthesis of inorganic nanomaterials [92]. She and his coworker also suggest that microwave radiation has been employed in organic and inorganic syntheses, oxidation/reduction reactions, and polymerizations [93]. Baek and his peer refer to the advantage of microwave radiation based on the high absorption of the GO compared to the solvent nanomaterials by soft chemistry [94]. This technique offers a more homogeneous heating process and can speed up the reaction rate by orders of magnitude. It can heat the reactant to a high temperature very quickly by transferring energy selectively to microwave absorbing polar solvents with a simultaneous increase in self-generated and metal oxide precursors. GO acts as the principal microwave absorber and can, therefore, be selectively heated, leading to the nucleation of the metal oxide onto its surface.
Generally, the precursor solution was ultrasonicated and magnetically stirred over a period of time. Afterward, the slurry was placed in a microwave oven under cyclic microwave radiation for several cycles. Cyclic microwave radiation was employed in order to avoid bumping. The procedure continues with centrifugation and drying of the product. Other previous works that use microwave approaches include the preparation of Ag/GO nanocomposites [95] and Mn3O4/rGO nanocomposites [93].
Room temperature synthesis
In this method, the metal salt solution in a specific solvent and the linker solution in the same/different solvent is prepared and mixed with stirring. The resultant solution is further stirred for longer hours on a magnetic stirrer and the product is separated by filtration, washed several times with the solvent, and dried at room temperature [96].
Impregnation method
The catalyst support is the material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts and nanomaterial-based catalysts occurs at the surface atoms. Consequently, great effort is made to maximize the surface area of a catalyst by distributing it over the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of carbon, alumina, silica, and organic polymer. In impregnation techniques, the support is contacted with a precursor solution, in other words, impregnation is related to ion exchange (adsorption processes) and the interaction with the support is dominant. Thus, low loadings, often for precious metals, are achieved by adsorption of the precursor molecules onto surface groups of the support (ion adsorption) or through the exchange of ions in, for example, zeolites (ion exchange), after which excess precursor is removed. When higher loadings are required, the washing step is skipped and the support is directly dried so that all precursor ends up on the support (impregnation and drying). Impregnation can be performed to incipient wetness, whereby the pores of the support are filled with precursor solution, to prevent deposition on the external surface of the catalyst grains and to limit waste [97].
Characterization of photocatalysts
There are different techniques to characterize photo-catalyst materials. The basic techniques are discussed below.
Ray Diffraction (XRD)
XRD is a non-destructive analytical technique that can be applied for the identification of unknown specimens and for the determination of materials properties. It is the most important and beneficial technique in solid state chemistry and it has been applied for the fingerprint characterization of crystals and for the determination of their structures. This method requires an X-ray source (monochromatic or of variable wavelength), the sample which is under investigation, and a detector that takes the diffracted X-rays [98].
Thermogravimetric Analysis (TGA)
Thermal analysis is the measurement of chemical and physical properties of materials as a function of temperature and thermogravimetric analysis is one of the types of thermal analysis. Thermogravimetry is a method in order to measure the change in weight of a substance as a function of temperature or time and results are given as a continuous chart record. In this method, a few milligrams of the sample are weighed and heated at a constant rate in the range from 1 to 20 oC min-1. The sample has a constant weight until it starts to decompose at the initial temperature. Decomposition generally occurs over a range of temperatures, from the initial temperature to the final temperature and a second constant weight plateau is observed above the final temperature that corresponds to the final weight of the sample. Initial weight, final weight, and the difference between them are the basic properties of the sample and they are used for quantitative calculations of compositional changes. On the other hand, initial and final temperatures are based on the heating rate, the nature of the solid, and the atmosphere above the sample [99].
Scanning Electron Microscopy (SEM)
SEM is a type of electron microscope that provides information about a sample’s surface topography, composition, and other surface properties such as electrical conductivity. In SEM, it is possible to observe and characterize the heterogeneous organic and inorganic materials on a nanometer (nm) to micrometer (µm) scale [98]. A three-dimensional-like image of the surfaces of a very wide range of materials can be taken. The basic constituents of the SEM are the lens system, electron gun, electron collector, visual and photo-recording cathode ray tubes (CRTs), and associated electronics. In the scanning electron microscope technique, the electrons from a focused beam are rastered across the surface of the material. Then, electrons reflected by the surface of the sample and emitted secondary electrons are detected in order to give the surface topography of samples like catalysts, polymers, and crystals. It is a common method for examining particle size, magnetic domains, crystal morphology, and surface defects [100].
N2 adsorption and BET analysis
Porous solids are classified as microporous, mesoporous, and macroporous based on the size of their pores. Solids that have a pore size of 2 nm or below are known as microporous. The mesoporous solids are in the range of between 2 nm and 50 nm and above 50 nm and are known as macroporous (99 Negash Getachew, 2013). BET theory explains the adsorption of gas molecules on solid surfaces. It serves as the basis for the measurement of the specific surface area of a material. The concept used in this theory is the multilayer adsorption of molecules. Gas adsorption is the technique used for total surface area measurements. Gas molecules are condensed onto the pores of the sample. Depending on the amount of gas adsorbed, the resultant sample pressure is recorded. From this, the surface area can be calculated [101].
Fourier Transform Infrared Spectroscopy (FTIR)
Fourier transform infrared spectroscopy (FTIR) is a technique that is used to obtain an infrared spectrum of absorption or emission of solid, liquid, or gas. An FTIR spectrometer simultaneously collects high spectral resolution data over a wide spectral range. It is ideal for the identification of functional groups present within a sample. FTIR was used to characterize the presence of specific functional groups such as C = O, -NO2, C - N and C - F; just to name a few, are all associated with characteristic infrared absorptions in the materials. The presence of different functional groups in as-synthesized nanomaterials was characterized by FTIR [102].
Photoluminescence (PL)
Spectroscopic and electroanalytical techniques are used to evaluate the efficiency of charge separation and characterization of the heterostructure. PL spectra helped us to trace the fates of the photogenerated electron/hole pairs. For an effective photocatalytic reaction the photogenerated electron/hole pair should follow the first path, and thus, should not recombine. PL spectroscopy concerns monitoring the light emitted from atoms or molecules after they have absorbed photons [103]. It is suitable for materials that exhibit photoluminescence. PL spectroscopy is suitable for the characterization of both organic and inorganic materials of virtually any size, and the samples can be in solid, liquid, or gaseous forms. The sample’s PL emission properties are characterized by four parameters: intensity, emission wavelength, the bandwidth of the emission peak, and emission stability [104]. The emission intensity of the as-synthesized nanocomposites was characterized by PL.
Wide research on nanomaterials is ongoing in the field of nano photocatalysts. Although many developments in nano photocatalytic materials occurred, still some important inquiries are required related to the characteristics of nano photocatalytic materials. The synthesis of significant structures such as nanorod, nanosphere, nanoflowers, nanoflakes, and nanocones with enhanced functional and structural properties could be opened an extensive area of study.
The synthesis of novel nano photocatalysts with excellent efficiency, inexpensive, eco-friendly, and high stability is crucially needed.
The heterogeneous photocatalytic for wastewater remediation is inhibited by some main technical problems that need to be studied effectively. Finally, a significant photo-catalytic treatment with better solar-driven, excellent efficacy, and fewer site area requirements can be comprehended in the future with fast assessment.
Heterogeneous photocatalysis has been proven to carry significant potential for the degradation of organic compounds, bacteria, and microorganisms, as well as the reduction of toxic metal ions present in water and wastewater. The environmentally friendly nature of this technique makes it a promising candidate for these applications. Furthermore, many photocatalytic materials have been studied on various potential contaminants in the past three decades, and some of them have been proven useful in visible light too. However, so far, this technique has mainly been studied in laboratories, and not appreciably implemented in industries. It is suggested that future studies should not only focus on the materials and other parameters, but also on the photocatalytic reactor design.
Semiconductor photocatalytic technology using either UV light or solar has become more prominent owing to its advantages of the use of vast organic/inorganic pollutants and its mineralization aspects. Different water hazardous contaminants, like pesticides, herbicides and detergents are effectively removed by this photocatalytic process. The applicability of the heterogeneous photocatalytic technology for water treatment is constrained by several key technical issues that need to be further investigated.
In order to promote the feasibility of photocatalytic water treatment technology in the near future, several key technical constraints ranging from catalyst development to reactor design and process optimization have to be addressed. These include (i) catalyst improvement for a high photo-efficiency that can utilize wider solar spectra; (ii) catalyst immobilization strategy to provide a cost-effective solid-liquid separation; (iii) new integrated or coupling system for enhanced photo mineralization and (iv) effective design of photocatalytic reactor system optimized parameters.
Finally, a large-scale photocatalytic treatment process with high efficacy, solar-driven, and low site area requirements can be realized in the short future with a rapid evaluation of different possible pilot plant configurations.
The author would like to acknowledge Mr. Hirpo Hisence Dube for reviewing this paper and providing his feedback. The author is also indebted to the College of Natural and Computational Sciences and the Department of Chemistry at Mekdela Amba University for their contributions in the process of developing the review and provision of various services as well as for providing me a great opportunity to advance intellectually.
- Han F, Kambala VSR, Srinivasan M, Rajarathnam D. Naidu R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Applied Catalysis A. 2009; 359: 25-40.
- Gogate PR, Pandit AB. A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Advance in environmental research. 2004; 8: 501-551.
- Chen H, Zhao J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption. 2009; 15(4): 381-389.
- Ong ST, Keng PS, Lee WN, Ha ST, Hung YT. Dye waste water treatment. Water review. 2011; 3: 157-176.
- Huo SH, Yan XP. Metal-organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. Journal of Materials Chemistry. 2012; 22:7449-7455.
- Brown MA, De Vito SC. Predicting azo dye toxicity. Critical Reviews in Environmental Science and Technology. 1993; 23: 249-324.
- Kornaros M. Lyberatos G. Biological treatment of wastewaters from a dye manufacturing company using a trickling filter. Journal of Hazardous Material. 2006; 136: 95 102.
- Chen C, Ma W. Zhao J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chemical Society Reviews. 2010; 39(11): 4206-4219.
- Kubacka A, Fernandez-Garcia M, Colon G. Advanced nano-architectures for solar photocatalytic applications. Chemical Reviews. 2011; 112(3): 1555-1614.
- Dolbecq A, Mialane P, Keita B, Nadjo L. Polyoxometalate-based materials for efficient solar and visible light harvesting: application to the photocatalytic degradation of azo dyes. Journal of Materials Chemistry. 2012; 22(47): 24509-24521.
- Fan W, Zhang Q, Wang Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Physical Chemistry Chemical Physics. 2013; 15(8): 2632-2649.
- Anandan S, Vinu A, Mori T, Gokulakrishnan N, Srinivasu P, Murugesan V, Ariga K. Photocatalytic degradation of 2, 4, 6-trichlorophenol using lanthanum doped ZnO in aqueous suspension. Catalysis Communications. 2007; 8(9): 1377-1382.
- Hoffmann MR, Martin ST, Choi W,Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chemical Reviews. 1995; 95(1): 69-96.
- Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y. Visible-light photocatalysis in Nitrogen-Doped Titanium Oxides. Science. 2001; 293: 269-271.
- Bi Y, Ouyang S, Cao J, Ye J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X = Cl, Br, I) heterocrystals with enhanced photocatalytic properties and stabilities. Phys Chem Chem Phys. 2011 Jun 7;13(21):10071-5. doi: 10.1039/c1cp20488b. Epub 2011 Apr 26. PMID: 21519619.
- Montini T, Gombac V, Hameed A, Felisari L, Adami G, Fornasiero P. Synthesis, characterization and photocatalytic performance of transition metal tungstates. Chemical Physics Letter. 2010; 498: 113-119.
- Bi Y, Hu H, Ouyang S, Lu G, Cao J, Ye J. Photocatalytic and photoelectric properties of cubic Ag3PO4 sub-microcrystals with sharp corners and edges. Chem Commun (Camb). 2012 Apr 18;48(31):3748-50. doi: 10.1039/c2cc30363a. Epub 2012 Mar 8. PMID: 22398441.
- Ran J, Yu JG, Jaroniec M. Ni(OH)2 modified CdS nanorods for highly efficient visible-light-driven photocatalytic H2 generation. Green Chemistry. 2011; 13: 2708-2713.
- Wang H, Zhang L, Chen Z, Hu J, Li S, Wang Z, Liu J, Wang X. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev. 2014 Aug 7;43(15):5234-44. doi: 10.1039/c4cs00126e. PMID: 24841176.
- Dana D, Vlasta B, Mazur M, Malati MA. Investigations of metal-doped titanium dioxide photocatalysts. Applied Catalysis. 2002; 37: 91-105.
- Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol. 2009 Oct;107(4):1193-201. doi: 10.1111/j.1365-2672.2009.04303.x. Epub 2009 Apr 17. PMID: 19486396.
- Wolderufael T, Yadav OP, Taddesse AM. Synthesis, characterization and photocatalytic activity of AgN-codoped ZnO nanoparticles towards methyl red degradation. Bulletin Chemical Society of Ethiopia. 2013; 27: 221-232.
- Nibret A, Yadav OP, Diaz I, Taddesse AM. Cr-N Co-doped ZnO nanoparticles: synthesis, characterization and photocatalytic activity for degradation of Thymol blue. Bulletin Chemical Society Ethiopia. 2015; 29(2): 247-258.
- Tedla H, Diaz I, Kebede T, Taddesse AM. Synthesis, characterization and photocatalytic activity of zeolite supported ZnO/Fe2O3/MnO2 nanocomposite. Journal of Environmental Chemical Engineering. 2015; 3: 1586-1591.
- Shamaila S, Sajjad AK, Chen F, Zhang J. WO3/BiOCl, a novel heterojunction as visible light photocatalyst. J Colloid Interface Sci. 2011 Apr 15;356(2):465-72. doi: 10.1016/j.jcis.2011.01.015. Epub 2011 Jan 11. PMID: 21320705.
- Wang YQ, Gu B, Xu WL. Electro-catalytic degradation of phenol on several metal-oxide anodes. J Hazard Mater. 2009 Mar 15;162(2-3):1159-64. doi: 10.1016/j.jhazmat.2008.05.164. Epub 2008 Jun 27. PMID: 18684560.
- Stylidi M, Kondarides DI, Verykios XE. Visible light-induced photocatalytic degradation of Acid Orange 7 in aqueous TiO2 suspensions. Applied Catalysis B: Environmental. 2004; 47 (3): 189-201.
- Colmenares JC, Aramendia MA, Marinas A, Marinas JM, Urbano FJ. Synthesis, characterization and photocatalytic activity of different metal doped Titania systems. Applied Catalysis. 2006; 306: 120-127.
- Curco D, Gimenez J, Addardak A, Cervera-March S, Esplugas S. Effects of radiation absorption and catalyst concentration on the photocatalytic degradation of pollutants. Catalysis Today. 2002; 76: 177-188.
- Palmisan G, Addam M, Augugliar V, Caronna T, Di Paola A, Lopez EG, Lodd V, Marci G, Palmisan, L, Schiavell M. Selectivity of hydroxyl radical in the partial oxidation of aromatic compounds in heterogeneous photocatalysis. Catalysis Today. 2007; 122: 118-127.
- Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37-8. doi: 10.1038/238037a0. PMID: 12635268.
- Malato S, Ferna P, Maldonado M, Blanco J, Gernjak W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catalo Today 2009; 147: 1-59.
- Anta J. Electron transport in nano structured metal oxide semiconductors. Current Opinion Colloid Interface Science. 2012; 17: 124-131.
- Nozik A, Beard M, Luther J, Law M, Ellingson R, Johnson J. Semiconductor quantum dots and quantum dot arrays and application of multiple excitation generation to their degeneration photovoltaic solar cells. Chemical Review. 2010; 110: 6873-6890.
- Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005 Dec 15;172(12):1487-90. doi: 10.1164/rccm.200504-613PP. Epub 2005 Sep 8. PMID: 16151040; PMCID: PMC2718451.
- Nakata K, Fujishima A. TiO2 photocatalysis: design and applications. Journal of Photochemistry and Photobiology C. 2012; 13: 169-189.
- Cao Y, He T, Zhao L, Wang E, Yang W. Structure and phase transition behavior of Sn4+-doped TiO2 nanoparticles. Journal of Physical Chemistry C. 2009; 113: 18121-18124.
- Mao C, Zhao Y, Qiu X, Zhu J, Burda C. Synthesis, characterization and computational study of nitrogen-doped CeO2 nanoparticles with visible-light activity. Phys Chem Chem Phys. 2008 Sep 28;10(36):5633-8. doi: 10.1039/b805915b. Epub 2008 Jul 30. PMID: 18956099.
- Marschall R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Advanced Functional Materials. 2014; 24: 2421-2440.
- Kamat, P. Native and surface modified semiconductor nanocluster, Progress in inorganic chemistry. In: Karlin D Progress in Inorganic Chemistry: Molecular Level Artificial Photosynthetic Materials, John Wiley and Son, Hoboken, NJ. 1997; 44: 273-343.
- Beydoun D, Ama R, Low G, Mc Evoy S. Role of nanoparticles in photocatalysis. Journal Nanoparticles. 1999; 1: 439-458.
- Moniz SJA, Shevlin SA, Martin DJ, Guo ZX, Tang J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environmental Science. 2015; 8: 731-759.
- Li H, Zhou Y, Tu W, Ye J, Zou Z. State‐of‐the‐Art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Advanced Functional Materials 2015; 25: 998-1013.
- Kamat PV. Electrocatalytically active graphene-platinum nanocomposite. Chemistry of Pearson Education. 2015; 93: 267-300.
- Kim IY, Jo YK, Lee JM, Wang L, Hwang SJ. Unique advantages of exfoliated 2D nanosheets for tailoring the functionalities of nanocomposites. Journal of Physics Chemicals Letter. 2014; 5: 4149-4161.
- Wang Y, Wang Q, Zhan X, Wang F, Safdar M, He J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: a review. Nanoscale. 2013 Sep 21;5(18):8326-39. doi: 10.1039/c3nr01577g. PMID: 23873075.
- Yang ZM, Huang GF, Huang WQ, Wei JM, Yan XG, Liu, YY. Novel Ag3PO4/CeO2 composite with high efficiency and stability for photocatalytic applications. Journal of Materials Chemistry A. 2014; 2: 1750-1756.
- Lin H, Ye H, Xu B, Cao J, Chen S. Ag3PO4 quantum dot sensitized BiPO4: a novel p-n junction Ag3PO4/BiPO4 with enhanced visible-light photocatalytic activity. Catalysis Communications. 2013; 37: 55-59.
- Yang L, Luo S, Li Y, Xiao Y, Kang Q, Cai Q. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst. Environ Sci Technol. 2010 Oct 1;44(19):7641-6. doi: 10.1021/es101711k. PMID: 20831154.
- Zhao FM, Pan L, Wang S , Deng Q, Zou JJ, Wang L, Zhang X. Ag3PO4/TiO2 composite for efficient photodegradation of organic pollutants under visible light. Applied Surface Science. 2014; 317: 833-838.
- Jung S, Yong K. Fabrication of CuO-ZnO nanowires on a stainless steel mesh for highly efficient photocatalytic applications. Chem Commun (Camb). 2011 Mar 7;47(9):2643-5. doi: 10.1039/c0cc04985a. Epub 2011 Jan 13. PMID: 21234467.
- Helaïli N, Bessekhouad Y, Bouguelia A, Trari M. p-Cu2O/n-ZnO heterojunction applied to visible light Orange II degradation. Solar Energy. 2010; 84: 1187-1192.
- Marschall R, Wang L. Non-metal doping of transition metal oxides for visible light photocatalysis. Catalysis Today. 2014; 225: 111-135.
- Abi M, Tigabu Bekele T, Diaz I, Adgo A. Polyaniline supported CdS/CeO2/Ag3PO4 nanocomposite: An A-B type tandem n-n heterojunctions with enhanced photocatalytic activity. Journal of Photochemistry and Photobiology, A: Chemistry. 2021; 406: 113005.
- Rodwihok C, Wongratanaphisan D, TaM TV, Choi WM, Hur SH, Chung JS. Cerium-oxide-nanoparticle-decorated zinc oxide with enhanced photocatalytic degradation of methyl orange. Applied Science. 2020; 10: 1697.
- Chen F, Ho P, Ran R, Chen W, Si Z, Wu X, Weng D, Huang Z, Lee C. Synergistic effect of CeO2 modified TiO2 photocatalyst on the enhancement of visible light photocatalytic performance. Journal of Alloys and Compounds, doi: 10.1016/j.jallcom.2017;04:138.
- Asi MA, He C, Su M, Xia D, Lin L, Deng H, Xiong Y, Qiu R,Li XZ. Photocatalytic reduction of CO2 to hydrocarbons using AgBr/TiO2 nanocomposites under visible light. Catalysis Today. 2011; 175: 256-263.
- Li G, Lian Z, Wang W, Zhang D, Li H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy. 2016; 19: 446-454.
- Wu W, Zhang S, Xiao X, Zhou J, Ren F, Sun L, Jiang C. Controllable synthesis, magnetic properties, and enhanced photocatalytic activity of spindlelike mesoporous α-Fe(2)O(3)/ZnO core-shell heterostructures. ACS Appl Mater Interfaces. 2012 Jul 25;4(7):3602-9. doi: 10.1021/am300669a. Epub 2012 Jun 21. PMID: 22692878.
- Yuan Y, Huang GF, Hu WY, Xiong DN, Zhou BX, Chang S, Huang WQ. Construction of g-C3N4/CeO2/ZnO ternary photocatalysts with enhanced photocatalytic performance. Journal of Physics and Chemistry of Solids. 2017; 106: 1-9.
- Prabhu S, Viswanathan T, Jothivenkatachalam K. Jeganathan K. Visible light photocatalytic activity of CeO2-ZnO-TiO2 composites for the degradation of Rhodamine B. Indian Journal of Materials Science, doi.org/10.1155/2014/536123. 2014.
- Lee YL, Chi CF Liau SY. CdS/CdSe Co-Sensitized TiO2 photoelectrode for efficient hydrogen generation in a photoelectrochemical cell. Chemical Materials, 22: 922-927.
- Zhou, P., Yu, J. and Jaroniec, M. 2014. All‐solid‐state Z‐scheme photocatalytic systems. Advanced Materials. 2010; 26: 4920-4935.
- Iwase A, Ng YH, Ishiguro Y, Kudo A, Amal R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J Am Chem Soc. 2011 Jul 27;133(29):11054-7. doi: 10.1021/ja203296z. Epub 2011 Jul 6. PMID: 21711031.
- He Y, Zhang L, Teng B, Fan M. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ Sci Technol. 2015 Jan 6;49(1):649-56. doi: 10.1021/es5046309. Epub 2014 Dec 17. PMID: 25485763.
- Yu J, Wang S, Low J, Xiao W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys Chem Chem Phys. 2013 Oct 21;15(39):16883-90. doi: 10.1039/c3cp53131g. Epub 2013 Sep 3. PMID: 23999576.
- Miseki Y, Fujiyoshi S, Gunji T, Sayama, K. Photocatalytic water splitting under visible light utilizing I3−/I− and IO3−/I− redox mediators by Z-scheme system using surface treated PtOx/WO3 as O2 evolution photocatalyst. Catalysis Science Technology. 2013; 3: 1750-1756.
- Abe R, Shinmei K, Koumura N, Hara K, Ohtani B. Visible-light-induced water splitting based on two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J Am Chem Soc. 2013 Nov 13;135(45):16872-84. doi: 10.1021/ja4048637. Epub 2013 Oct 29. PMID: 24128384.
- Sasaki Y, Iwase A, Kato H, Kudo, A. The effect of Co-Catalyst for Z-scheme photocatalysis systems with a Fe3+/Fe2+ electron mediator on overall water splitting under visible light irradiation. Journal of Catalysis. 2008; 259: 133-137.
- Sayama K, Abe R, Arakawa H, Sugihara H. Decomposition of water into H2 and O2 by a two-step photoexcitation reaction over a Pt-TiO2 photocatalyst in NaNO2 and Na2CO3 aqueous solution. Catalysis Communication. 2006; 7: 96-99.
- Kato H, Hori M, Konta R, Shimodaira Y, Kudo, A. Construction of Z-scheme type heterogeneous photocatalysis systems for water splitting into H2 and O2 under visible light irradiation. Chemical Letters. 2004; 33(10): 1348-1349.
- Sasaki Y, Kato H, Kudo A. [Co(bpy)3]3+/2+ and [Co(phen)3]3+/2+ electron mediators for overall water splitting under sunlight irradiation using Z-scheme photocatalyst system. Journal of American Chemical Society. 2013;135: 5441-5449.
- Yun HJ, Lee H, Kim ND, Lee DM, Yu S, Yi J. A combination of two visible-light responsive photocatalysts for achieving the Z-scheme in the solid state. ACS Nano. 2011 May 24;5(5):4084-90. doi: 10.1021/nn2006738. Epub 2011 Apr 21. PMID: 21500836.
- Wang X, Li S, Ma Y, Yu H, Yu J. H2WO4·H2O/Ag/AgCl composite Nanoplates: A Plasmonic Z-scheme visible-light photocatalyst. Journal of Physical Chemistry C. 2011; 115(30), 14648-14655.
- Chen Z, Wang W, Zhang Z, Fang X. High-efficiency visible-light-driven Ag3PO4/AgI photocatalysts: Z-scheme photocatalytic mechanism for their enhanced photocatalytic activity. Journal of Physical Chemistry C. 2013; 117: 19346-19352.
- Ahmed S, Rasual MG,Brown R, Hashib MA. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenol contaminants in waste water. Journal of Environmental Management. 2010; 9: 311-330.
- Wu W, Changzhong Jiang, Roy VA. Recent progress in magnetic iron oxide-semiconductor composite nanomaterials as promising photocatalysts. Nanoscale. 2015 Jan 7;7(1):38-58. doi: 10.1039/c4nr04244a. PMID: 25406760.
- Zhang L, Jimei M. Nano phase materials and nanostructure. Beijing: Science press. 2001; 140-144.
- Chong MN, Jin B, Chow CW, Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010 May;44(10):2997-3027. doi: 10.1016/j.watres.2010.02.039. Epub 2010 Mar 18. PMID: 20378145.
- Shankar MV, Anandan S, Venkatachalam N, Arabindoo B, Murugesan V. Novel thin-film reactor for photocatalytic degradation of pesticides in aqueous solutions. Journal of Chemical Technology and Biotechnology. 2004; 79: 1279-1285.
- Sobczynski A, Duczmal L, Zmudzinski W. Phenol destruction by Solutions. Journal of Chemical Technology and Biotechnology. 2004; 79: 1279-1285.
- Mahalakshmi M, Vishnu Priya S, Arabindoo B, Palanichamy M, Murugesan V. Photocatalytic degradation of aqueous propoxur solution using TiO2 and Hbeta zeolite-supported TiO2. J Hazard Mater. 2009 Jan 15;161(1):336-43. doi: 10.1016/j.jhazmat.2008.03.098. Epub 2008 Mar 29. PMID: 18455297.
- Zhu C, Wang L, Kong L, Yang X, Wang L, Zheng S, Chen F, MaiZhi F, Zong H. Photocatalytic degradation of AZO dyes by supported TiO2 + UV in aqueous solution. Chemosphere. 2000 Aug;41(3):303-9. doi: 10.1016/s0045-6535(99)00487-7. PMID: 11057591.
- Jing HP, Wang CC, Zhang YW, Wang P, Li R. Photocatalytic degradation of methylene blue in ZIF-8. Journal of the Royal Society of Chemistry. 2014; 4: 54454-54462.
- Ayllon JA, A Figueras, S, Garelik L, Spirkova J, Durand L. Preparation of TiO2 powder using titanium isopropoxide decomposition in plasma enhanced chemical vapour deposition reactor. Journal of Material Science. 1999; 18: 1319-1321.
- Ohtani B, Ogawa Y, Nishimoto S. Photocatalytic activity of amorphous anatase mixture of titanium (IV) oxide particles suspended in aqueous solutions. Journal of Physical Chemistry. 1997; 101: 3746-3752.
- Kashif N, Ouyang F. Parameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2. J Environ Sci (China). 2009;21(4):527-33. doi: 10.1016/s1001-0742(08)62303-7. PMID: 19634430.
- Mikaela G. Metal organic frameworks for heterogeneous catalysis: synthesis and characterization. Dissertation, Stockholm University, Sweden. 2012.
- Vakiti RJ. Hydro/Solvothermal synthesis, structures and properties of metal-organic frameworks based on s-block metals, masters theses and specialist projects. Western Kentucky University, Bowling Green, Kentucky, paper. 2012; 1168.
- Faraji Yamini Y, Rezaee M. Magnetic nanoparticles: Synthesis, stabilization, functionalization, characterization, and applications. Journal of Iranian Chemical Society 2010; 7(1): 1-37.
- Abdollahi A, Abdullah H, Zainal Z, Yusof NA. Synthesis and characterization of manganese doped ZnO nanoparticles. International Journal of Basic and Applied Sciences. 2011; 11(4): 62-68.
- Lu H. Interfacial synthesis of metal-organic frameworks. Open Access Dissertations and Theses. 2012; 7188.
- She X, Zhang X, Liu J, Li L, Yu X, Huang Z, Shang, S. Microwave-assisted synthesis of Mn3O4 nanoparticles/reduced graphene oxide nanocomposites for high performance supercapacitors. Materials Research Bulletin. 2015; 70: 945-950.
- Baek S, Yu SH, Park S K, Pucci A. Marichy C, Lee DC, Pinna N. A one-pot microwave-assisted non-aqueous sol-gel approach to metal oxide/graphene nanocomposite for Li-ion batteries. RSC Advances. 2011; 1(9): 1687-1690.
- Li Q,Hai P. Rapid microwave-assisted synthesis of silver decorated-reduced graphene oxide nanoparticles with enhanced photocatalytic activity under visible light. Materials Science in Semiconductor Processing. 2014; 22: 16-20.
- Huang L, Wang H, Chen J, Wang Z, Sun J, Zhao D, Yan Y. Synthesis, morphology control and properties of porous metal organic coordination polymers (MOCP). Journal of Microporous and Mesoporous Materials 2003; 58: 105-114.
- Munnik P, Petra E, De Jongh, Krijn P. De Jong- inorganic Chemistry Review. 2015; 115: 6687-671.
- Ece O. Hydrothermal synthesis and structural characterization of open-frame work metal phosphates template with organic diamine. Unpublished MSc Thesis, Izmir Institute of Technology. 2012.
- Negash Getachew. Benign methods for the synthesis of metal organic frameworks (MOFs), Unpublished doctoral dissertation, Addis Ababa University, Addis Ababa, Ethiopia. 2013.
- Smart L, Moore EA. Solid state chemistry. Taylor and Francis, New York. 2005.
- Mohanty RP. Fabrication and characterization of metal-organic framework (MOF) based membrane. Unpublished BSc thesis. National Institute of Technology, Rourkela. Molecular Catalysis 2012; 213: 225-230.
- Khan SA, Khan SB, Khan LU, Farooq A, Akhtar K, M Asiri M. Fourier transforms infrared spectroscopy: fundamentals and application in functional groups and nanomaterials characterization. Handbook of Materials Characterization. 2018;317-344. DOI:10.1007/978-3-319-92955-2_9.
- Gfroerer TH. Photoluminescence in analysis of surfaces and interfaces. Davidson College, Davidson, USA. John Wiley and Sons, Ltd. DOI: 10.1002/9780470027318.a2510. 2006.
- Qu Y, Duan X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem Soc Rev. 2013 Apr 7;42(7):2568-80. doi: 10.1039/c2cs35355e. PMID: 23192101.