More Information
Submitted: March 18, 2021 | Approved: April 01, 2021 | Published: April 02, 2021
How to cite this article: de Souza ACA, de Filippi MCC, Nascente AS, Prabhu AS, Alves E. Silicon rates and beneficial microorganism on blast suppression and productivity of upland rice. J Plant Sci Phytopathol. 2021; 5: 020-027.
DOI: 10.29328/journal.jpsp.1001057
Copyright License: © 2021 de Souza ACA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Oryza sativa L., Bioagent; Nutrition; Silicate; Resistance; Grain production
Silicon rates and beneficial microorganism on blast suppression and productivity of upland rice
Alan Carlos Alves de Souza1*, Marta Cristina Corsi de Filippi2, Adriano Stephan Nascente2, Anne Sitarama Prabhu2 and Eduardo Alves1
1Department of Phytopathology, Federal University of Lavras, Lavras MG, Brazil
2EMBRAPA Rice and Beans, Santo Antônio de Goiás-GO, Brazil
*Address for Correspondence: Alan Carlos Alves de Souza, Department of Phytopathology, Federal University of Lavras, Lavras, MG, Brazil, Email: [email protected]
One of the primary constraints in upland rice cultivation is the disease blast (Magnaporthe oryzae), which can provide reduction up to 100% of the grain yield The use of silicon with beneficial microorganisms (bioagents) can be an alternative for the control of this disease and to provide an increase in the productivity of the rice grain. The objective of this work was to study the effect of rates of silicon with bioagents in blast suppression and grain yield of upland rice. The methodology used was tests carried out in field conditions, in two different areas: Capivara and Palmital farms, during the growing season 2015/2016. The experimental design was in a split-plot scheme with four replications. In the main plots were the silicon fertilization rates (0, 2, 4 and 8 ton ha-1) and in the subplots was the bioagents (1-without bioagents, 2-Pseudomonas fluorescens, 3-Burkholderia pyrrocinia, 4-Trichoderma asperellum, 5-a mixture of the three bioagents). The results showed that the use of 2 ton ha-1 of silicon with a mixture of bioagents was the best treatment to control leaf blast. Besides, from rates, 2 to 6 ton ha-1 of silicon in Capivara Farm and up to 8 ton ha-1 of silicon in Palmital Farm provided the highest grain yield. A mixture of bioagents provided the highest grain yield. In this sense, it was concluded that the best recommendation to connect blast control, grain yield and reduced amount of silicon was the use of 2 ton ha-1 of silicon with the mixture of bioagents.
Rice is a crop grown throughout the world, occupying, in the year 2016 an area around 160 million hectares, with an annual production of around 741 million Mg of paddy grains. China is the world's largest rice producer, contributing about 28.3% of world production [1]. Globally, Brazil ranks ninth in the production of the rice grain, with an estimated production for 2017/2018 of 12 million Mg in an area of almost 2 million ha [2]. Rice is a crop that is easily adaptable to different soil and climate conditions, and because of this, it is the species with the most significant potential to increase production and to fight against global hunger [3-5].
In Brazil, rice can be grown in two types of ecosystems: the lowland and upland condition with supplemental irrigation or rainwater only [6]. In the upland ecosystem, rice is usually planted in rotation with soybeans, in the renewal of degraded pastures, or as the first crop in opening up new areas for agriculture [7]. The production of upland rice is growing in global importance due to the reduction of irrigation water availability for irrigated lowland rice crops [8].
Among the factors that can provide a reduction in upland rice grain yield, there are diseases. Rice blast disease caused by Magnaporthe oryzae B. Couch [Pyricularia grisea (Cooke) Sacc.] is a significant constraint on worldwide rice production [9]. This disease is very aggressive and in the tropical region can cause yield losses of up to 100% [10]. In this sense, to avoid yield loss by blast farmers apply a high amount of fungicides [11-13]. The use of fungicides has efficiency in blast control; however, its use as the only strategy to control this disease can cause environmental contamination, increase pathogen resistance to different active principles, besides increasing the costs of rice production [14]. Therefore, there is a need to search for alternatives to control rice blast in a sustainable way that avoids environmental polluting, reduce the production cost, increase grain yield and allow improving profits for the farmers. The use of silicon and beneficial microorganisms, such as plant growth-promoting rhizobacteria (PGPR) and Trichoderma spp. fungus, has been highlighted in researches as sustainable alternatives, because they present high efficiency in controlling diseases, improving plant development and increasing rice grain yield [8,14-21].
Silicon is considered the second most abundant element in the Earth's crust and is absorbed by the roots of the plants as monossilicic acid (H4SiO4), accumulating in different vegetal tissues, mainly of the wall of foliar cells [22]. The use of silicon in agriculture offers several benefits to the plants, such as resistance to diseases and pests, higher resistance to drought, salinity, and contributes to the improvement of nutritional status [23]. In resistance to diseases, silicon can act in a passive way, when the accumulation of silicon in the cell wall acts as a physical barrier to the penetration of pathogens and, actively, when the element is able to activate genes involved in the production of antimicrobial compounds [16-24,25].
Plant growth-promoting rhizobacteria and Trichoderma fungus also bring several benefits to plants, such as disease resistance, growth promotion, atmospheric nitrogen fixation, solubilization, and nutrient availability, siderophores production and plant growth regulating hormones [20,26,27] (Figure 1). As a biocontrol, these bioagents act by different mechanisms of action, such as antibiosis, parasitism, production of antimicrobial compounds and also induction of resistance [13,21,28,29].
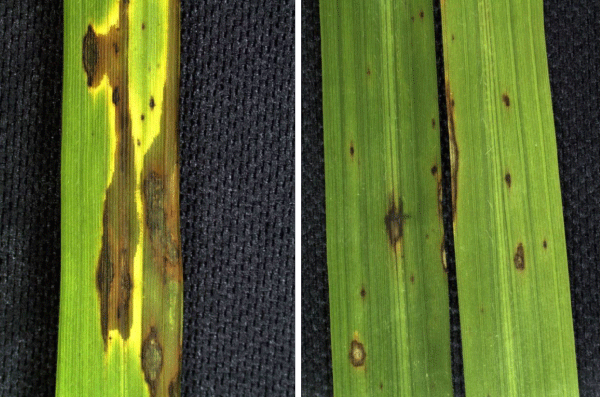
Figure 1: Symptoms of leaf blast in rice plants not nourished with silicon (A) and nourished with silicon (B).
However, there is still a lack of information on the use of microorganisms associated with silicon in agriculture and specifically in rice crops. There are a large number of species that inhabit the rhizosphere of plants, and therefore a large number of interactions could occur between microorganisms, silicon, and plants. For example, it is estimated that there are approximately 30,000 different species of bacteria and only 8% of them have been identified [30]. Thus, there are several interactions among silicon, plant, and microorganisms that are not yet known and, if investigated, can bring benefits to agricultural systems [26]. In this sense, the objective of this work was to study the effect of rates of silicon with bioagents in blast suppression and grain yield of upland rice.
Site description
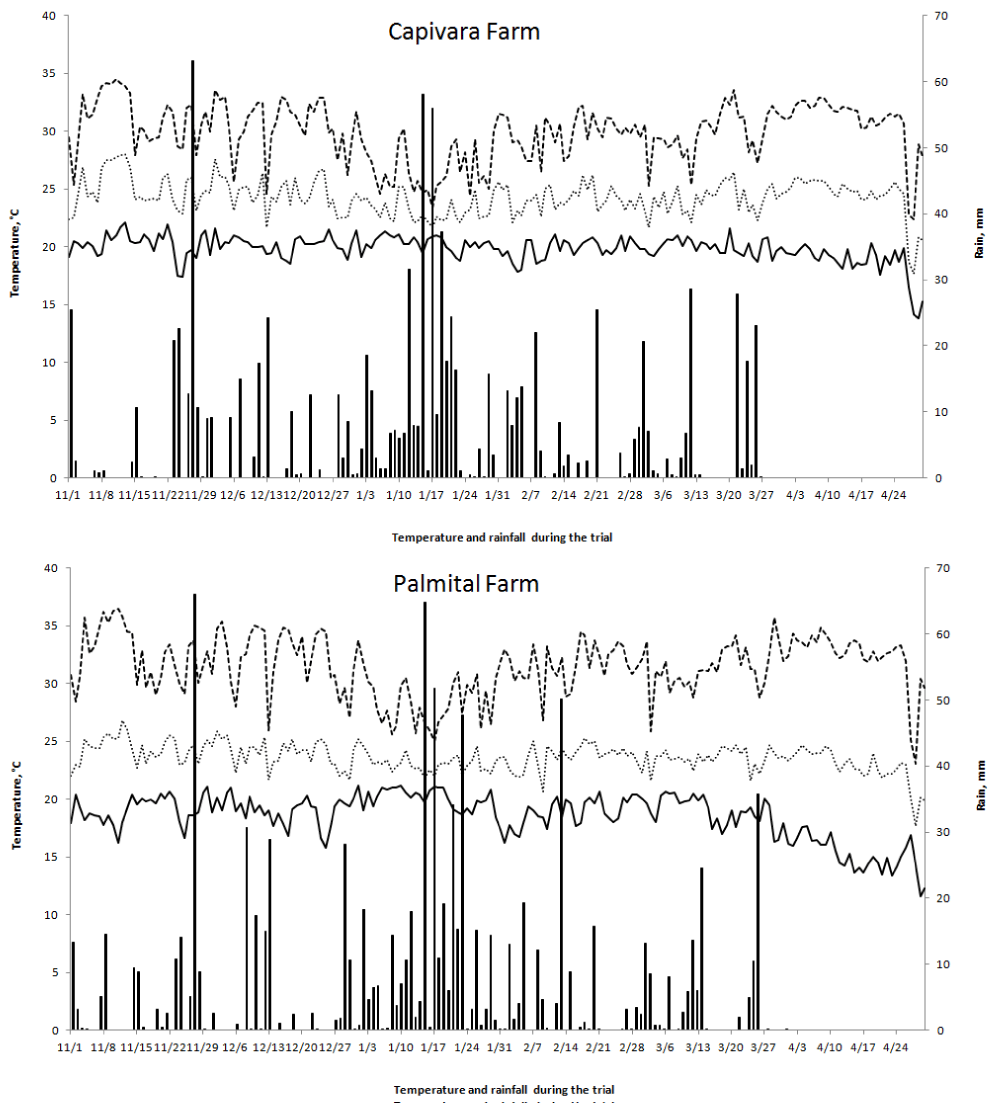
The experiments were located in the Capivara and Palmital Farms. The Capivara Farm is located in Santo Antônio de Goiás, GO, Brazil, at 16º28'00" S and 49 º17'00" W, and 823 m of altitude. The Palmital Farm is located in the municipality of Goianira, GO, Brazil, at 16°26'14" S, 49°23'50" W, and 720 m of altitude. The climate is tropical savanna, considered Aw according to the Köppen classification [31]). There are two distinct seasons, usually dry from May to September (fall/winter) and rainy from October to April (spring/summer). During the experiment, the temperature and amount of rainfall data were recorded (Figure 2).
Figure 2: Maximum (T maximum), minimum (T minimum) and average (T average) temperatures and rainfall during the trial period of upland rice grown under a no-tillage system in the experimental fields of Capivara and Palmital farms in the 2015/16 growing season.
In both places soil was managed under no-tillage system for five years with millet as last cultivated crop. The soil in both locations was classified as a sandy clay loam (kaolinitic, thermic Typic Haplorthox) and was corrected by liming. Before the application of treatments, the chemical characteristics of the soil were determined according to the methods described by Donagema, et al. (2011). Capivara farm had the following results pH CaCl2: 5.42; clay: 400.0 g kg-1; silt: 180.0 g kg-1; sand: 420.0 g kg-1; K+: 100.0; P: 15.13; Ca: 3.0; Mg: 1.20; Al+3: 0.0; Si: 2.25 mg dm-3) and Palmital farm had pH CaCl2: 5.22; clay: 440.0; silt: 160.0; sand: 400.0; K+: 156.0; P: 6.17; Ca: 2.80; Mg: 1.0; Al+3: 0.0; Si: 2.62 mg dm-3).
Experimental design and treatments
Trials were conducted in rainfed conditions using the genotype of rice BRS Primavera, which is considered susceptible to blast [32]. Trials were arranged in a randomized block, arranged in a split-plot scheme, with four replications. Treatments consisted (main plots) of four silicon rates [1 - 0 Mg ha-1 (no silicon), 2 - 2.0 Mg ha-1, 3 – 4.0 Mg ha-1 and 4 - 8.0 Mg ha-1] and five (subplots) bioagents species [1 – no bioagents, 2 - Pseudomonas fluorescens, 3 - Burkholderia pyrrocinia, 4 – Poll of Trichoderma asperellum (T06, T09, T12, T56), 5 - mixture of three bioagents]. The main plots had a size of 100 m2 (4 x 25 m) and subplots 20 m2 (4 x 5 m). Each subplot consisted of eight 5-m long rice rows, spaced at 0.5 m. Data samples were collected in four central rows, and 1 m from the end of each plant row and two external rows constituted the edge.
Rhizobacteria P. fluorescens (R-46) and B. pyrrocinia (R-55) belong to the Microorganism Culture Collection at the Embrapa Rice and Bean Research Center [13]. The pool of T. asperellum isolates belongs to the Fungi Collection of the Plant Protection Laboratory of the Federal Rural University of Amazônia [33].
Calcium and magnesium silicate (SiCaMg) was used as the silicon source, containing 10.5% silicon, 27% calcium, 6% magnesium and 95% of useful calcium carbonate equivalence. Levels of calcium and magnesium in the soil were balanced, within each silicon rate tested, with the use of dolomitic limestone. Application of silicon and limming was made 30 days before rice sowing.
Application of bioagents
Three types of applications of bioagents in rice plants were carried out: seed (microbiolization), soil and foliar spraying. For the applications via seed and soil, the B. pyrrocinia bioagents and the T. asperellum pool were used, and for the foliar application, P. fluorescens was used, according to Fillipi, et al., França, et al. [13,27].
B. pyrrocinia suspension was prepared with water, from cultures that had been growing for 24 hours on solid medium 523 [34], at 28 °C, and the concentration was set in a spectrophotometer to A540 = 0.5 (108 UFC). Rice seeds were immersed in the bacterial suspension, during 24 hours under constant agitation at 25 °C, and control seeds (bacteria-free) were immersed only in water, during 24 hours, in the same conditions according to Sperandio, et al. [21].
Each isolate of T. asperellum pool was grown in a Petri dish containing PDA (potato dextrose and agar) for 5 days and formulated as described by Silva, et al. [33]. The seed treatment was performed at concentrations of 10 g of powdered [33] T. asperellum per 1 kg of seed [13,33]. The concentration of the biological suspension was 108 conidia ml-1. Soil application of B. pyrrocinia (108 UFC) or T. asperellum pool (108 conidia ml-1) was made at 15 days after sowing, with suspension prepared as described above, in the same concentration, at 200 l ha-1, with a costal atomizer, according to Filippi, et al. [13].
P. fluorescens solution for plant spray was done as the same as B. pyrrocinia. Plant spray pulverization of P. fluorescens suspension was sprayed on the plants, using a manual backpack sprayer, at a constant pressure provided by a CO2 pressure source, and a conical nozzle type (TX-VS2), at 15 days after sowing with a volume of 200 L ha-1, according to Filippi, et al. [13].
Rice crop management
Rice was cultivated in the no-tillage system. So, millet was desiccated with a glyphosate application (1.8 kg ha-1 acid equivalent) 40 days before sowing of the upland rice. Fertilization at sowing furrows was calculated according to the soil’s chemical characteristics [35]. Therefore, sowing fertilization was 20 kg ha-1 of N as urea, 120 kg ha-1 of P2O5 as triple superphosphate and 60 kg ha-1 of K2O as potassium chloride, in both places. Nitrogen topdressing fertilization (as urea) was carried out 45 days after the rice emergence, using 60 kg ha-1 of N. Rice sowing was performed mechanically using 200 seeds m-2, on December 15th, 2015 (Capivara farm) and on January 14th, 2016 (Palmital farm). Rice plant emergence occurred on average five days after sowing for both places. Cultural practices were performed according to standard rice crop recommendations, keeping crop free from weeds, diseases, and insects.
Evaluations
The severity of leaf blast and panicles: For the evaluation of leaf blast, it was sampled five times in each plot in periods of 48 hours. It was sampled five rice tillers per subplots in each time, to estimate the area under the disease progress (AUDPC). For the evaluation of the panicle blast, 50 panicles were collected per subplot, at 80 days after sowing, in the maturing of the grains stage. In both evaluations, the samples were collected and taken to the Laboratory of Technical Support of Embrapa Rice and Beans for the quantification of the blast disease. The quantification of leaf blast severity was performed using a scanner, where rice plants were scanned in high-resolution image (200 dpi) and QUANT® disease quantification program, obtaining the percentage of diseased and healthy area. For the severity of the panicle blast, a five-note scale (1, 3, 5, 7 and 9) was used, taking into account the percentage of empty spikelets in the panicle, and the incidence of neck blast (panicle base) [36].
Silicon content in rice leaves: Rice leaf samples were collected from the flag leaf at the full blooming stage. Leaves from 50 plants per plot were collected, washed and then dried under forced-air circulation at 65 °C for 72 h before grinding and analyzing the samples for chemical composition. Besides, in each plot, one sample of 100 g of grains was taken for nutrient analyses. The concentration of Si was determined using methods described by Korndorfer, et al. [37].
Shoot dry biomass: Rice shoots of 1.0 m from one of the rows, in each subplot, were sampled at the full blooming stage. The shoots were washed in water and then dried in an oven with forced air circulation at 65 ºC, then weighed to determine the dry shoot mass.
Grain yield: Rice harvest was carried out by hand after physiological maturity (March 3rd, 2016 Capivara Farm and April 29th, 2016, Palmital Farm) of the grains in the usable area of each subplot. Grain yield was determined by weighing the harvested grain of each subplot, corrected to 13% of the water content and converted to kg ha-1.
Statistical analysis
For statistical analysis, the Statistical Package for the Social Sciences (SPSS), version 21.0. In the qualitative factor (with or without bioagents), data were subjected to an analysis of variance, and when the F test proved significant, the data were compared by a Tukey test at p < 0.10. In the quantitative factor (Si rates), results were submitted to regression analysis when p < 0.10. Plots, subplots, and all subplots interactions were considered as random effects. The Si rates, use of bioagents and places were considered as fixed effects.
Leaf blast severity
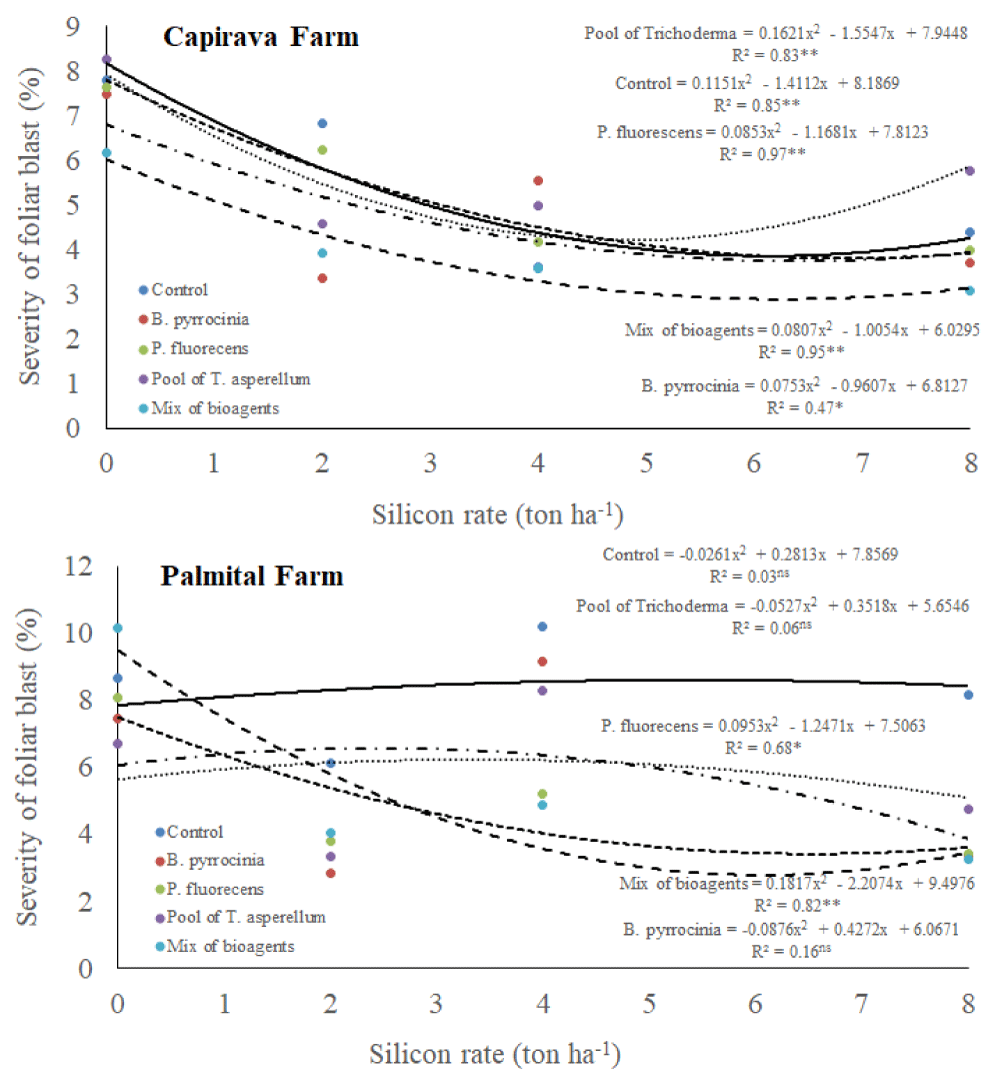
There was a triple interaction among silicon rates, bioagents and places for leaf blast severity (Table 1). In general, the severity of blast disease was higher in Palmital Farm than in Capivara Farm (Figure 3). At Capivara Farm, in all treatments, datas were explained by quadratic regressions, with tendencies for less rice blast severity when increasing silicon rates. However, a mixture of bioagents provided the lowest leaf blast severity. At Palmital Farm, increasing silicon rates did not provide a reduction in the rice blast severity at the control treatment (no bioagents). On the other hand, for all bioagents treatments, datas fited in quadratic regressions, with reducing values of rice blast severity when increasing silicon rates.
| Table 1: F values of isolated and interaction factors for severity of foliar blast (SFB), area below disease progression curve (AUDPC), severity of neck blast (SNB), biomass (Biomass), Foliar silicon content (Si Foliar) and grain yield (GY). | ||||||
| Factors | SFB | AUDPC | SNB | Si Foliar | Biomass | Yield |
| Silicon (Si) | 0.000 | 0.000 | 0.021 | 0.000 | 0.033 | 0.000 |
| Bioagents (Bio) | 0.000 | 0.100 | 0.985 | 0.000 | 0.855 | 0.000 |
| Places (P) | 0.000 | 0.000 | 0.000 | 0.000 | 0.100 | 0.044 |
| Si x Bio | 0.004 | 0.277 | 0.912 | 0.000 | 0.981 | 0.137 |
| Si x P | 0.000 | 0.090 | 0.051 | 0.000 | 0.000 | 0.000 |
| Bio x P | 0.000 | 0.250 | 0.846 | 0.000 | 0.485 | 0.413 |
| Si x Bio x P | 0.006 | 0.380 | 0.941 | 0.000 | 0.502 | 0.251 |
| +Not significant by the F test at p < 0.10. | ||||||
Figure 3: Severity (%) of leaf blast, on upland rice plants grown under no-tillage system, as a function of silicon rates and bioagents at Capivara and Palmital farms. Growing season 2015/16.
Area under disease progres curve (AUDPC)
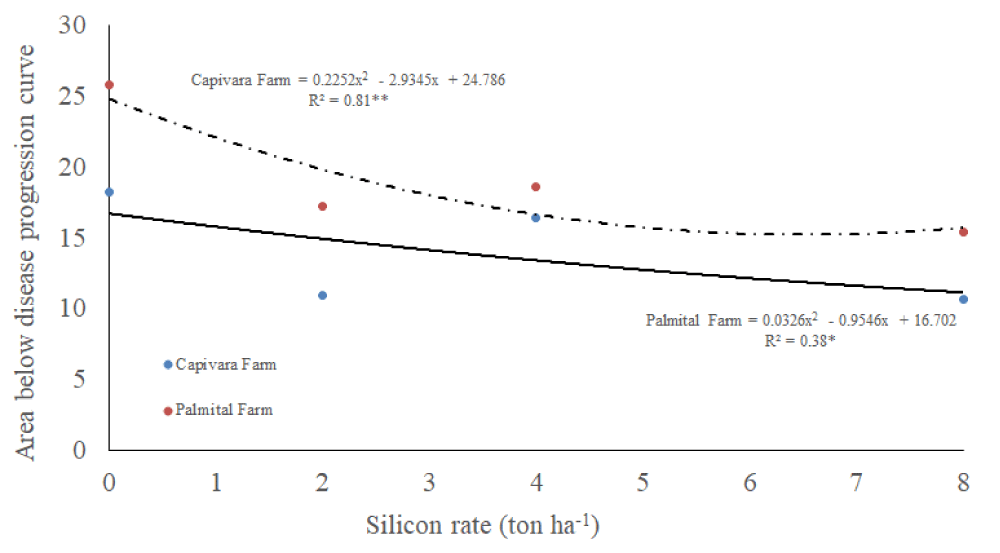
Concerning AUDPC, there was an interaction between silicon rates and places, and a single effect of bioagents (Table 1). It was observed that, increasing silicon rates, reduced AUDPC, in both farms. AUPDC values were higher in Palmital Farm than in Capivara Farm (Figure 4). Regarding bioagents, all treatments with them allowed lower values of AUDPC and differed from the control (no bioagents) (Table 2).
Figure 4: Area below disease progression curve on upland rice plants grown under no-tillage system, as a function of silicon rates at Capivara and Palmital farms. Growing season 2015/16.
| Table 2: Area below disease progression curve (AUDPC) in upland rice plants grown under no-tillage system due to the use of bioagents. | |
| Bioagent | AUDPC |
| Control (no bioagent) | 18.92 a++ |
| B. pyrrocinia | 16.66 b |
| P. fluorecens | 15.58 b |
| Pool of T. asperellum | 16.80 b |
| Mixture of bioagents+ | 14.23 b |
| +Means mixture of B. pyrrocinia + P. fluorecens + pool of T. asperellum. ++Means followed by the same letter, do not differ for Tukey test at p < 0.10. | |
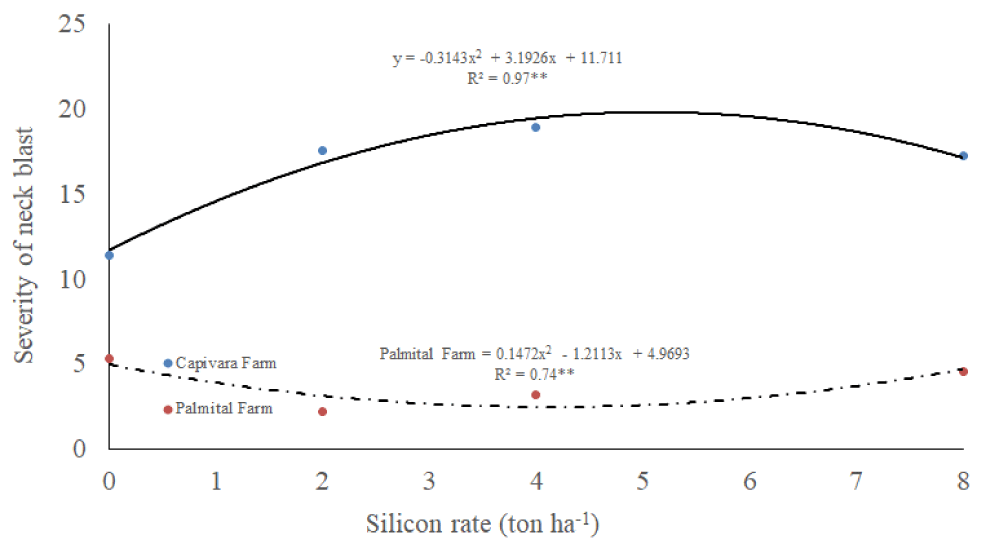
Severity of neck blast
There was an interaction between silicon rates and places for the severity of neck blast (Table 1). The severity of neck blast was higher in Capivara Farm than in Palmital Farm (Figure 5). In the Capivara and Palmital Farm, the treatment composed of plants fertilized with 2 ton ha-1 of silicon presented a lower neck blast severity compared to the control.
Figure 5: Severity of neck blast on upland rice plants grown under no-tillage system, as a function of silicon rates at Capivara and Palmital farms. Growing season 2015/16.
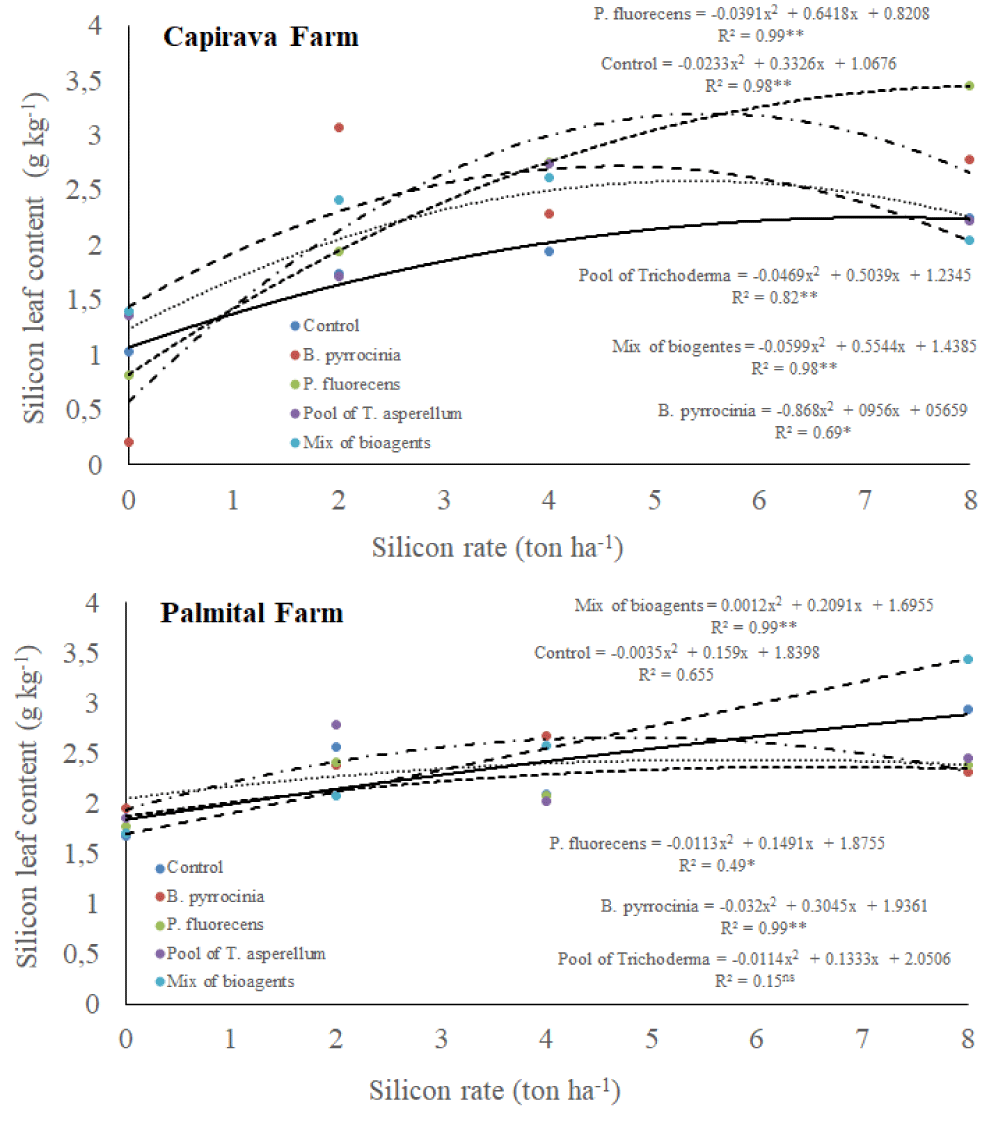
Silicon content in rice leaves
There was a triple interaction among silicon rates, bioagents and places for silicon content in rice leaves (Table 1). In both farms, there were tendencies to silicon content in rice leaves by increasing silicon rates (Figure 6). At Capivara Farm, the silicon contents in rice leaves in treatment composed of 8 ton ha-1 of silicon and the bioagent P. fluore scence was highlighted concerning the control treatment (without bioagents). At Palmital Farm, the silicon contents in rice leaves in the treatment composed of 8 ton ha-1 of silicon with the bioagents mixture was significantly higher than the control treatment.
Figure 6: Silicon leaf content on upland rice plants grown under no-tillage system, as a function of silicon rates and bioagents at Capivara and Palmital farms. Growing season 2015/16.
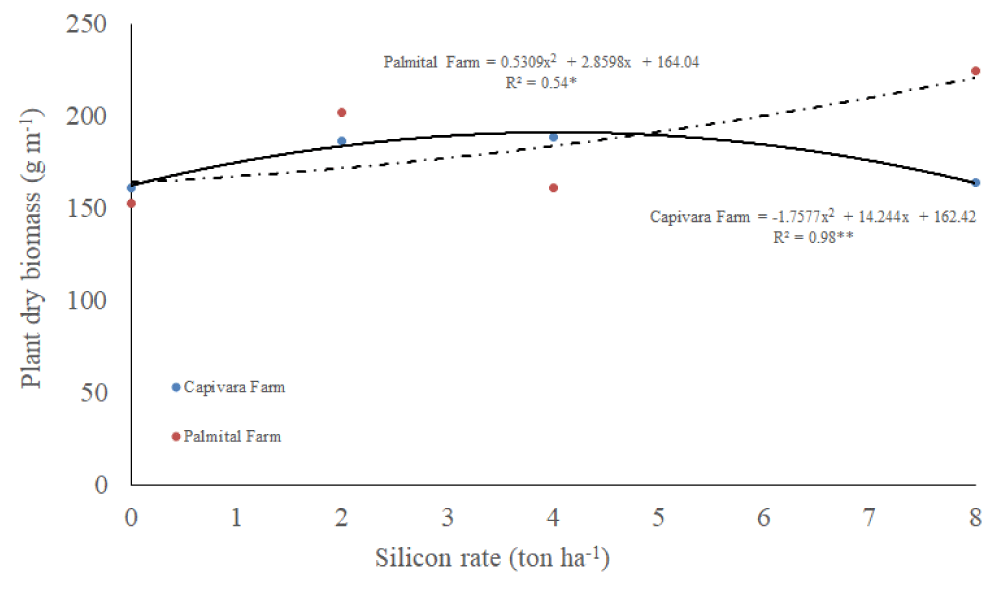
Dry biomass
There was an interaction between silicon rates and places for the dry mass of upland rice plants (Table 1). At Capivara Farm, dates fitted in a quadratic regression, dry mass increased up until 6 ton ha-1 and then, reduced the values (Figure 7). At Palmital Farm, dates also fitted in a quadratic regression, however increasing silicon rates, increased dry biomass.
Figure 7: Plant dry biomass on upland rice plants grown under no-tillage system, as a function of silicon rates at Capivara and Palmital farms. Growing season 2015/16.
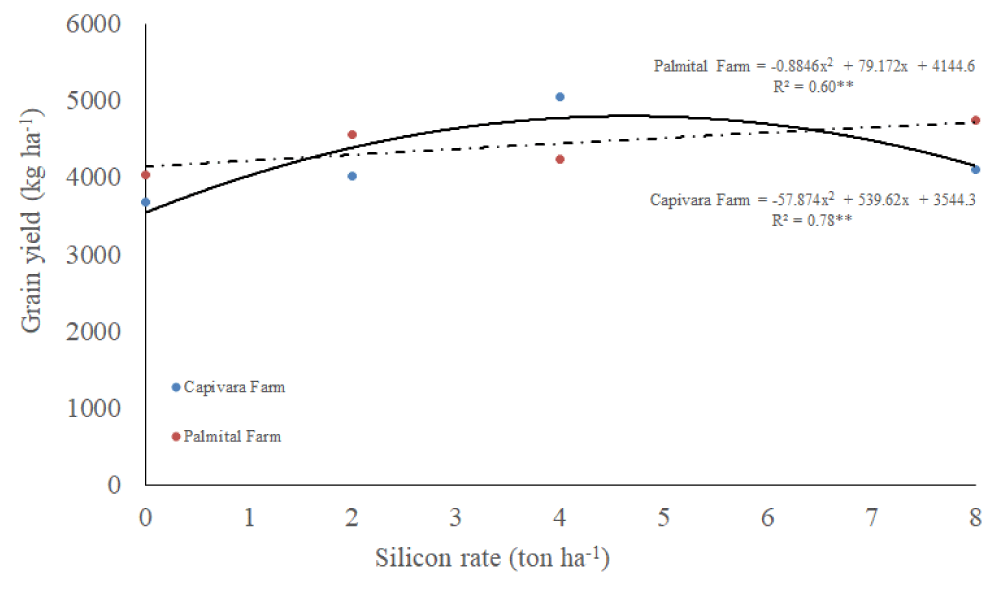
Grain yield
There was an interaction between silicon rates and places, and a single effect of bioagents for grain yield (Table 1). It was observed that grain yield improved by increasing silicon rates up to 6 ton ha-1 in Capivara Farm (Figure 8). On the other hand, in Palmital Farm, grain yield increased as increased silicon rates. Treatment with a mixture of bioagents allowed the highest upland rice grain yield and differed from all other treatments (Table 3). Control treatment had the lowest grain yield and differed from all other treatments.
Figure 8: Grain yield of upland rice grown under no-tillage system, as a function of silicon rates at Capivara and Palmital farms. Growing season 2015/16.
| Table 3: Grain yield of upland rice plants grown under no-tillage system due to the use of bioagents. | |
| Bioagent | Grain yield (kg ha-1) |
| Control (no bioagent) | 3877 c |
| B. pyrrocinia | 4335 b |
| P. fluorecens | 4399 b |
| Pool of T. asperellum | 4157 b |
| Mixture of bioagents+ | 4821 a |
| +Means mixture of B. pyrrocinia + P. fluorecens + pool of T. asperellum. ++Means followed by the same letter, do not differ for Tukey test at p < 0.10. | |
The two places in which the experiments were conducted differed, concerning the severity of the blast disease in the rice plants. Capivara Farm (576 mm of rain and 23.8 ºC average temperature during the trial, Figure 2) was sowed on December 15th, 2015, and presented minor blast disease severity in relation to Palmital Farm (576.6 mm of rain and 23.3 ºC average temperature during the trial, Figure 2), which was sowed on January 14, 2016. According to Prabhu, et al. [22] and Robert and Walker, [38] in areas with similar environmental and agricultural conditions, blast pressure may vary when cultivated at different times, with a higher incidence of blast diseases in later sowing.
The combination of 2 tons ha-1 of silicon with a mix of bioagents provided less than 4% of the severity of leaf blast in the trial conducted at Capivara Farm. In the Palmital Farm, the combination of 2 ton ha-1 of silicon with B. pyrrocinia, P. fluorecens, the pool of T. asperellum and the mix of all bioagents provided less than 5% of leaf blast severity. Therefore, we could see that the combination of silicon and bioagents was sufficient to control leaf blast. The use of bioagents and the use of silicon in rice plants had demonstrated efficiency in the control of blast and other diseases [39-43]. Corroborating with this information, Cortes, et al. (2015) observed rice plants treated with the combination of 2 ton ha-1 of silicon with B. pyrrocinia, or with and T. asperellum, suppressed 92% and 93% of the blast disease, when compared the control treatment, under greenhouse conditions. Bueno, et al. [44], also showed, under greenhouse conditions, that 2 ton ha-1 of silicon combined with B. pyrrocinia suppressed 55% of leaf scald in rice plants.
Many studies have shown that silicon prevents and/or delays the penetration of the pathogen through the formation of a mechanical barrier, composed of amorphous silica, depoused between the cuticle layer and the epidermis layer, in foliar cells, and also acts by systematically inducing the production of defense enzymes and proteins [45-48]. Other studies showed that bioagents act effectively on plant resistance to diseases, being able to control pathogens by antibiosis, parasitism, by competition of nutrients, producing enzymes that degrade pathogen structures and together activating latent defense mechanisms, inducing resistance in plants [28,29,49].
In our trial, another factor could potentiate the absorption and the effect of silicon, on the plants, by the bioagents to control leaf blast. Some sources of silicon, such as silicates, have in their composition much of the silicon in the polymerized form. According to Liu, et al. [50], PGPR's applied to soil act in the rhizosphere by degrading particles of the polymerized mineral through the excretion of polysaccharides, making the silicon available to the plants and potentializing their absorption. According to Curry and Perry [51], this absorption and silicon accumulation in the leaves can be regulated by an active process, which is stimulated and activated in the plant by protection against different stress conditions, among them the attack of pathogens. For example, Bueno, et al. [44] showed that rice plants fertilized with silicon, treated with B. pyrrocinia-treated, and inoculated with Monographella albescens, increased foliar silicon concentration and disease resistance, when compared to the control treatment (without silicon and bioagent) by 3 times. These affirmations corroborate to our findings on the content of foliar silicon, as applying silicon in the soil increases the foliar silicon content. Besides, the area below the disease progression curve was reduced by the use of silicon and with all the bioagents tested.
Concerning the effect of silicon rates at Capivara Farm, the dose of 4 ton. ha-¹ showed an increase of 27% of grain yield, when compared to the control (without silicon), representing a difference of 27.4 bags (50 Kg) per hectare. In the same area, the 17.4% increase in plant biomass of the same treatment was also observed concerning the control. At Palmital Farm, the treatment that stood out was the 8 ton ha-1 of silicon, which provided an increase in 17.5% in grain yield when compared to the control, representing a difference of 14 bags per hectare. In this area, silicon fertilization also influenced the increase in biomass. According to Polleto, et al. (2011), the increased productivity provided by the use of silicon can be explained by improved availability of nutrients to plants, increased biomass, growth promotion and resistance to biotic stresses, mainly to diseases. Corroboration with this information, Ramos, et al. [53] used carbonized rice hull as a source of silicon in upland rice and observed an increase of 16% in grain yield, concerning the silicon-free treatment. Santos, et al. [51], similarly to the present study, recorded a increase of 50% in rice yield using 4 ton ha-1 of silicon. Sousa, et al. [55], in a greenhouse, reported a 37% of gain in the total growth of rice plants fertilized with 2 ton ha-1 of silicon, when compared to the silicon-free plants. According to Carvalho-Pupatto, et al. [56], plants fertilized with silicon increase the root system from 30 to 50 cm, allowing the plants to exploit higher volume of soil, increasing the absorption of nutrients, both with higher mobility and nitrogen, responsible for the photosynthetic gain.
Regarding the effect of bioagents on rice grain yield, the treatment that stood out was the mixture of the three bioagents, presenting grain yield 19.6% higher than the control (without bioagents). The various benefits can explain the efficiency of these bioagents in increasing grain yield, such as the promotion of plant growth through increasing hormone’s levels, the fixation of atmospheric nitrogen and the solubilization of minerals [57]. Trichoderma and PGPR's directly affect hormone concentrations in the plant's rhizosphere, with an increasing demand for gibberellin, cytokinin, abscisic acid and ethylene, promoting the increase and improving root mass, the assimilation of nutrients and water and, consequently, increasing nutrients absorption of [57-59]. França, et al. [27], using a mixture of the same species of T. asperellum of the present work in irrigated rice, reported a 70% increase in grain yield concerning the control (without bioagent). Nascente, et al. [20] applying B. pyrrocinia and P. fluorescens in irrigated rice found higher concentrations of nitrogen, phosphorus, magnesium, copper, iron, and zinc concerning the control treatment. Rêgo, et al. [60], working with the mixture of the three bioagents of the present study, reported an increase of 14 % of roots and 27% of the diameter of the vascular cylinder of rice plants, when compared to the control treatment. Sousa, et al. [55] proved that the mixture of the three bioagents used in the present work in combination with 2 ton ha-1 of silicon increased by up to 68% the shoots, 37.04% the root system and 58, 38% in the total of rice plants biomass. According to the authors, this biomass improvement occurred due to the increase of sugar’s level, chlorophyll, gibberellin and salicylic acid contents in rice plants treated with bioagents and silicon, improving the germination, tillering and vigor of the plants, essential characteristics for plants of rice cultivated in the field, mainly in a no-tillage system.
Our data showed that the use of 2 ton ha-1 of silicon with a mixture of bioagents was the best treatment for controlling leaf blast. Besides, from rates, 2 to 6 ton ha-1 of silicon, in Capivara Farm, and up to 8 ton ha-1 of silicon in Palmital Farm, provided the highest grain yield. The mixture of bioagents provided the highest grain yield. In this sense, the best recommendation to connect blast control, grain yield and reduction of the amount of silicon, in the BRS Primavera rice variety, was the use of 2 ton ha-1 of silicon with the mixture of bioagents.
We appreciate the support provided EMBRAPA Rice and Beans, National Council for Scientific and Technological Development and Federal University of Lavras.
- FAO. Food and Agriculture Organization of the United Nations. Rice Market Moitor. 2018; 21.
- CONAB Companhia Nacional de Abastecimento. Acompanhamento da safra brasileira de grãos 2017/18. 2018; 5.
- CGIAR Science Council. IRRI's upland rice research follow-up review to the 6th IRRI EPMR. Science Council Secretariat. 2006.
- Africa Rice Center (WARDA). The Growing NERICA Boom in Uganda. WARDA Publications. 2009.
- Kumar V, Ladha JK. Direct seeding of rice: recent developments and future research needs Advances in Agronomy. 2011; 111: 297-396.
- Silva VAC, Silva EF, Tabosa JN. Comportamento de genótipos de arroz de terras altas na zona da mata de Pernambuco. Revista Brasileira de Engenharia Agrícola e Ambiental. 2010; 10: 1030-1037.
- Santos AB, Stone LF, Vieira NRA. A cultura do arroz no Brasil. 2.ed. Embrapa Arroz e Feijão. 2006; 1000.
- Nascente AS, Filippi MCC, Lanna AC, Sousa TP, Souza ACA, et al. Effects of beneficial microorganisms on lowland rice development. Environ. Sci Pollut Res. 2017; 24: 25233-25242. PubMed: https://pubmed.ncbi.nlm.nih.gov/28929284/
- Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol. 2016; 34: 147–153. PubMed: https://pubmed.ncbi.nlm.nih.gov/27816794/
- Prabhu AS, Filippi MC, Silva GB, Silva-Lobo VL, Morais OP. An Unprecedented Outbreak of Rice Blast on a Newly Released Cultivar BRS Colosso in Brazil. In: Wang GL, Valent B. (Eds.) Advances in Genetics, Genomics and Control of Rice Blast. Springer Science. 2009; 257-267.
- Prabhu AS, Filippi MCC. Brusone em arroz: controle genético, progresso e perspectivas. Santo Antonio de Goiás: Embrapa Arroz e Feijão. 2006; 388.
- Foley JA, Ramankutty N, Brauman K, Cassidy E, Gerber J, et al. Solutions for a Cultivated Planet. Nature. 2011; 478: 337-342.
- Filippi MCC, Silva GB, Silva-Lobo VL, Cortes MMCB, Moraes AJG, et al. Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biological Control. 2011; 58: 160-166.
- Filippi MCC, Silva GB, Cortes MVB, Silva-Lobo VL, Prabhu AS. Indução de resistência e promoção de crescimento em arroz por agentes biológicos. In: Rodrigues FA, Fortunato AA, Resende RS. Indução de resistência a patógenos: VI Reunião Brasileira Sobre Indução de Resistência em Plantas a Patógenos na Universidade Federal de Viçosa. 2012; 51-78
- Datnoff LE, Snyder GH, Korndorfer GH. Silicon in Agriculture. Elsevier. 2001; 403.
- Rodrigues FA, Jurick WM, Datnoff LE, Jones JB, Rollins JA. Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol Mol Plant Pathol. 2005; 66: 144-159.
- Souza ACA, Sousa TP, Cortês MVB, Rodrigues FA, Silva GB, et al. Enzyme induced defense response in the suppression of rice leaf blast (Magnaporthe oryzae) by silicon fertilization and bioagents. Int J Res Stu Biosc. 2015; 3: 22-32.
- Oliveira JC, Albuquerque GMR, Xavier AS, Mariano RLR, Suassuna ND, et al. Characterization of Xanthomonas citri subsp. malvacearum causing cotton angular leaf spot in Brazil. J Plant Pathol. 2011; 93: 707-712.
- Cruz MFA, Rodrigues FA, Polanco LR, Curvêlo CRS, Nascimento KJT, et al. Inducers of resistence and silicone on the activity of defense enzymes in the soybean-Phakopsora pachyrhizi interation. Bragantia. 2013; 72: 162-172.
- Nascente AS, Filippi MCC, Lanna AC, Souza ACA, Silva-Lobo VL, Silva GB. Biomass, gas exchange, and nutrient contents in upland rice plants affected by application forms of microorganism growth promoters. Environ. Sci. Pollut Res. 2017a; 24: 2956–2965. PubMed: https://pubmed.ncbi.nlm.nih.gov/27844322/
- Sperandio EM, Vale HMM, Reis MS, Cortes MVCB, Lanna AC, et al. Evaluation of rhizobacteria in upland rice in Brazil: growth promotion and interaction of induced defense responses against leaf blast (Magnaporthe oryzae). Acta Physiol Plant. 2017; 39: 258-270.
- Prabhu AS, Guimarães CM, Berni RF. Influência da época de plantio no controle da brusone em folhas de arroz de terras altas. Pesquisa em foco. 2000; 56.
- Menegale ML, Castro GSA, Mancuso MAC. Silício: interação com o sistema solo-planta. Journal of Agronomic Sciences. 2015; 4: 435-454.
- Gratão PL, Polle A, Lea PJ, Azevedo RA. Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol. 2005; 32: 481-494. PubMed: https://pubmed.ncbi.nlm.nih.gov/32689149/
- Sakr N. The role of silicon (Si) in increasing plant resistance against fungal diseases. Hell Plant Protect J. 2016; 9: 1–15.
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Uni. 2014; 26: 1–20.
- França SKS, Cardoso AF, Lustosa DC, Ramos EMLS, Filippi MCC, et al. Biocontrol of sheath blight by Trichoderma asperellum in tropical lowland rice. Agron Sustain Dev. 2015; 35: 317-324.
- Shoresh M, Yedidia I, Chet I. Involvement of Jasmonic Acid/Ethylene Signaling Pathway in the Systemic Resistance Induced in Cucumber by Trichoderma asperellum T203. Biological Control. 2005; 95: 76-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/18943839/
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, et al. Trichoderma-plant-pathogen interactions. Soil Biol Biochemy. 2008; 40: 1-10.
- Barea JM, Pozo MJ, Azcon R, Aguilar CA. Microbial co-operation in the rhizosphere. J Exp Bot. 2005; 56: 1761-1778. PubMed: https://pubmed.ncbi.nlm.nih.gov/15911555/
- Alvarez CA, Stape JL, Sentelhas PC, Moraes Gonçalves JL, Sparovek G. Köppen´s climate classification map of Brazil. Meteorologische Zeitschrift. 2014; 22: 711-728.
- Malavolta VMA, Azzini LE, Bastos CR, Salomon MV, Castro JL. Progresso da brusone nas folhas e panículas de genótipos de arroz de terras altas. Summa Phytopathologica. 2008; 34:186-188.
- Silva JC, Torres DB, Lustosa DC, Filippi MCC, Silva GB. Rice sheath blight biocontrol and growth promotion by Trichoderma isolates from the Amazon. Rev Ciênc Agrárias. 2012; 55: 243-250.
- Kado CI, Heskett MG. Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas and Xanthomonas. Phytopathology. 1970; 60: 969-76. PubMed: https://pubmed.ncbi.nlm.nih.gov/5469886/
- Sousa DMG, Lobato E. Cerrado: correção do solo e adubação 2. ed. Embrapa Informação Tecnológica. 2004; 416.
- EMBRAPA Centro Nacional de Pesquisa de Arroz e Feijão. Manual de métodos e pesquisa em arroz: 1ª aproximação. Goiânia. 1977; 106.
- Korndorfer GH, Pereira HS, Nolla A. Análise de silício: solo, planta e fertilizante. Uberlândia. GPSi-ICIAG-UFU. 2004; 34.
- Robert KM, Walker AJ. Na introduction to the physiology of crop yields. Longman Scientific e Technical. 1989; 8-15.
- Datnoff LE, Raid RN, Snyder GH, Jones DB. Effect of calcium silicate on blast and brown spot intensities and yields of rice. Plant Dis. 1991; 75: 729-732.
- Datnoff LE, Snyder GH, Deren CW. Influence of silicon fertilizer grades on blast and brown spot development and on rice yields. Plant Dis. 1992; 76: 1011-1013.
- Datnoff LE, Deren CW, Snyder GH. Silicon fertilization for disease management of rice in Florida. Crop Protection. 1997; 16: 525-531.
- Osuma-Canizales FJ, Dedatta SK, Bonman JM. Nitrogen form and silicon nutrition effects on resistance to blast disease of rice. Plant and Soil. 1991; 135: 223-231.
- Winslow MD. Silicon disease resistance and yield of rice genotypes under upland cultural conditions. Crop Science. 1992; 32: 1208-1213.
- Bueno ACSO, Castro GLS, Silva Junior DD, Pinheiro HA, Filippi MCC, et al. Response of photosynthesis and chlorophyll a fluorescence in leaf scald-infected rice under influence of rhizobacteria and silicon fertilizer. Plant Pathology. 2017; 66: 1487-1495.
- Santos GR, Korndorfer GH, Pelúzio JM, Didonet J, Reis Filho JCD, et al. Influência de fontes de silício sobre a incidência e severidade de doenças e produtividade do arroz irrigado. Biosci J. 2003; 19: 65-72.
- Santos LV, Prabhu AS, Ferreira E, Fageria NK. Fertilização silicatada na severidade de brusone e na incidência de insetos-praga em arroz irrigado. Revista Brasileira de Engenharia Agrícola e Ambiental. 2009; 13: 537-543.
- Rodrigues FA, Datnoff LE. Silicon and rice disease management. Fitopatologia Brasileira. 2005; 30: 457-470.
- Debona D, Rodrigues FA, Datnoff LE. Silicon’s Role in Abiotic and Biotic Plant Stresses. Ann Rev Phytopathol. 2017; 5: 85-107. PubMed: https://pubmed.ncbi.nlm.nih.gov/28504920/
- Van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopathol. 1998; 36: 453-483. PubMed: https://pubmed.ncbi.nlm.nih.gov/15012509/
- Liu WX, Xu XS, Wu XH, Yang QY, Luo YM, et al. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ Geochem Health. 2006; 28:133-140. PubMed: https://pubmed.ncbi.nlm.nih.gov/16528584/
- Currie HA, Perry CC. Silica in plants: biological, biochemical and chemical studies. Ann Bot. 2017; 100: 1383-1389. PubMed: https://pubmed.ncbi.nlm.nih.gov/17921489/
- Poletto N, Mundstock CM, Grohs DS, Mazurana M. Padrão de afilhamento em arroz afetado pela presença dos íons amônio e nitrato. Bragantia. 2011; 70: 96-103.
- Ramos LA, Korndorfer GH, Queiroz AA. Avaliação de fontes de silício em plantas de arroz do ecossistema de várzea. Biosci J. 2012; 25:10-16.
- Santos GR, Neto MDC, Rodrigues AC, Bonifacio A, Korndorfer GH. Fertilização silicatada e nitrogenada no controle da brusone do arroz em sistema irrigado. Revista Caatinga. 2014; 27: 103-108.
- Sousa TP, Souza ACA, Filippi MCC, Lanna AC, Cortês MVB, et al. Bioagents and silicon promoting fast early upland rice growth. Environ Sci Pollut Res. 2017.
- Carvalho Pupatto JG, Büll LT., Crusciol CAC. Atributos químicos do solo, crescimento radicular e produtividade do arroz de acordo com a aplicação de escórias. Pesquisa Agropecuária Brasileira. 2004; 39: 1213-1218.
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species- opportunistic, avirulent plant symbionts. Nat Rev. 2004; 2: 43-56. PubMed: https://pubmed.ncbi.nlm.nih.gov/15035008/
- Dodd SL, Crowhurst RN, Rodrigo AG, Samuels GJ, Hill RA, et al. Examination of Trichoderma phylogenies derived from ribosomal DNA sequence data. Mycol. Res. 2000; 104: 23-34.
- Drogue B, Dore H, Borland S, Wisniewski-Dyé F, Prigent-Combaret C. Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res. Microbiol. 2012; 163: 500-510. PubMed: https://pubmed.ncbi.nlm.nih.gov/22989671/
- Rêgo MCF, Ilkiu-Borges F, Filippi MCC, Gonçalves LA, Silva GB. Morphoanatomical and biochemical changes in the roots of rice plants induced by plant growth-promoting microorganisms. J Bot. 2014; 1–9.