More Information
Submitted: 10 April 2020 | Approved: 18 April 2020 | Published: 20 April 2020
How to cite this article: Abd El-Aziz MH. Natural infection of squash fruits (Cucurbita pepo) by Zucchini Yellow Mosaic potyvirus (ZYMV) in Alexandria governorate. J Plant Sci Phytopathol. 2020; 4: 028-032.
DOI: 10.29328/journal.jpsp.1001047
ORCiD ID: orcid.org/0000-0002-4604-4063
Copyright License: © 2020 Abd El-Aziz MH. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Natural infection of squash fruits (Cucurbita pepo) by Zucchini Yellow Mosaic potyvirus (ZYMV) in Alexandria governorate
Mahmoud Hamdy Abd El-Aziz*
Plant Pathology Institute, Agricultural Research Center, Alexandria, Egypt
*Address for Correspondence: Mahmoud Hamdy Abd El-Aziz, Plant Pathology Institute, Agricultural Research Center, Alexandria, Egypt, Tel: +201229556042; Email: [email protected]
An isolate of zucchini yellow mosaic virus (ZYMV) was obtained from naturally infected squash fruits were grown in Abees region, Alexandria governorate. Disease symptoms were Showing mosaic, yellowing and blistering and absis symptoms. The identification was based on the symptoms developed on diagnostic hosts and serological reactions with antisera to cucumber mosaic cucumovirus (CMV), watermelon mosaic potyvirus 2 (WMV-2) and ZYMV. Squash fruit isolate of ZYMV was transmitted by Aphis gossypii, Aphis neri and Myzus persicae in non-persistent manner. The virus was purified by ultra-centrifugation and PEG. The purified virus had an ultraviolet absorption spectrum typical of a nucleoprotein with A260/280 and A280/260 being 1.1 and 0.91 respectively. The yield of purified virus was 1.62 mg/100g infected leaf tissues. Specific antiserum was prepared and found to have a titer of 1:409600 as determined by indirect ELISA.
Zucchini yellow mosaic virus (ZYMV) is a member of family Potyviridae and is considered the most economically important virus attacking cucurbit plants under field conditions [1]. The virus was isolated for the first time in northern Italy [2] and latter from different countries [3-8]. In Egypt, ZYMV was isolated from naturally infected squash plants [9-15]. Squash plants exhibiting mosaic, yellowing, blister and stunt symptoms and squash fruits contain (abscess or Boils) were observed in Abees region, Alexandria governorate. The aims of present study was observed to isolate and identify the causal agent on the bases of symptomology, reactions of diagnostic hosts, serological reaction, purified the isolated virus by ultra-centrifugation methods and Measurement the yield of purified virus. Production of specific antiserum to the isolated virus was also carried out. The present study was directed to isolate and identify the causal agent on the bases of symptomology, reactions of diagnostic hosts, serological reaction, insect transmission, photometrical characters of the purified virus. Production of specific antiserum to the isolated virus was also carried out.
Fruit samples from squash plants exhibiting mosaic, blister and absis or Boils symptoms were collected from Abees location (Figure 1), Alexandria governorate. Inoculum was prepared by grinding infected leaf tissue in a mortar and pestle, with small amount of 0.02 M phosphate buffer, pH7.0, containing 0.1% 2-mercaptoethanol, leaves of plants to be inoculated were first dusted with carborundum (600 mesh) [16] and then inoculated with a freshly prepared inoculum using forefinger. The isolated virus was maintained in squash plants that served as a source of the virus for subsequent studies.
Figure 1: Fruit samples from naturally infected squash plants exhibiting mosaic, blister and absis or Boils.
Diagnostic hosts
The following diagnostic hosts; Chenopodium amaranticolor, cucumber (Cucumis sativum L.) c.v. Bita Alpha, melon (Cucumis melo L.) cv. Ananas, squash (Cucurbita pepo L.) c.v. Eskandrani, watermelon (Citrullus lanatus Thunb.) c.v. Giza 1, luffa (Luffa aegyptiaca Mill), Datura stramonium, Gomphrena globosa, Nicotiana glutinosa, N. rustica and Phaseolus vulgaris c.v. Bountiful were known to give characteristic symptoms for some viruses affecting cucurbits as recorded by Lisa, et al. [2], Lesemann, et al. [3], Provvidenti, et al. [17], Ibrahim, [10], Huang, et al. [18], Awad, et al. [11], Abdel Ghaffar, et al. [12], Prieto, et al. [19], Younes, [14], Fath-Allah and Ahmed, [15], Usher, et al. [20] and Vučurović et al. [8] were used as provisional identification of the isolated virus.
Modes of transmissions
ZYMV was studied for its transmissibility by different methods.
Mechanical transmission
Squash plants were infected and was used as a virus source, whereas, Chenopodium amaranticolor was an assay host. Hamza, et al. [16].
Aphid transmission
Three species of aphids were tested for their ability to transmit isolates of ZYMV. Apterous forms of three species of aphids; Aphis gossypii Glover, A. neri and Myzus persicae (These aphids were kindly identified by Dr. Hedaya Hamza, Entomology Departmet, Faculty of Agriculture, Alexandria University, Egypt). Apterous forms of aphids were starved for one hour then allowed to feed on ZYMV-infected squash leaves for 3-5 min. on infected squash attached leaves. Aphids were transferred in groups of five to each of 10 healthy squash plants and allowed an inoculation period of 10 min. before they were killed by spraying by an insecticide. Inoculated plants were kept under greenhouse conditions and symptoms were observed for four weeks [21].
Serological reaction
Source of antisera: Antisera to cucumber mosaic cucumovirus (CMV) [22], Zucchini Yellow Mosaic potyvirus (ZYMV) was supplied by [14] and the antiserum of watermelon mosaic potyvirus 2 (WMV-2) antiserum was provided by Agdia.
Indirect ELISA: Indirect ELISA was carried out as described by Abd El-Aziz and Younes, [23] extracts from infected and healthy squash fruits were diluted with coating buffer (0.05 M carbonate, pH 9.6) to 1:10. Wells were coated with antigens by adding 100 µl of to the bottom of the well and incubated for 3 hours at 37 °C or overnight at 4°C. The plates were rinsed three times by flooding wells with (Phosphate buffer saline tween 20 (PBST)) for 3 minutes each. Antisera requiring cross-adsorption were diluted 1:500 with filtered extract from healthy tissues diluted 1:20 [24] in serum buffer (Phosphate buffer saline tween 20 (PBS-T) containing 2% soluble polyvinylpyrrolidone, 0.2% Bovine serum albumin (BSA)), and incubated for 45 min. at 37 °C. The precipitate, which had formed, was removed by centrifugation for 10 min. at 5000 rpm. 100 µl aliquots from the diluted antisera were added to each well, after which the plates were incubated at 37 °C for 2 hours or at 4 °C overnight, then washed as before. Goat anti-rabbit gamma globulin conjugated to alkaline phosphatase was diluted 1:15000 in serum buffer, and 100 µl were added to each well, followed by one hour incubation at 37 °C, and then washed as before. 100 µl of the enzyme substrate, 0.5 mg/ml paranitrophenyl phosphate in 10% diethanolamine buffer, pH 9.8 were added to each well and incubated at room temperature (25 °C) for about 30 minutes. The enzyme activity was stopped by adding 50 µl of 3 M NaOH. The ELISA values measured by MeterTech E960 ELISA reader were expressed as absorbency at 405 nm and optical density of at least double that of the healthy control were considered positive.
Purification
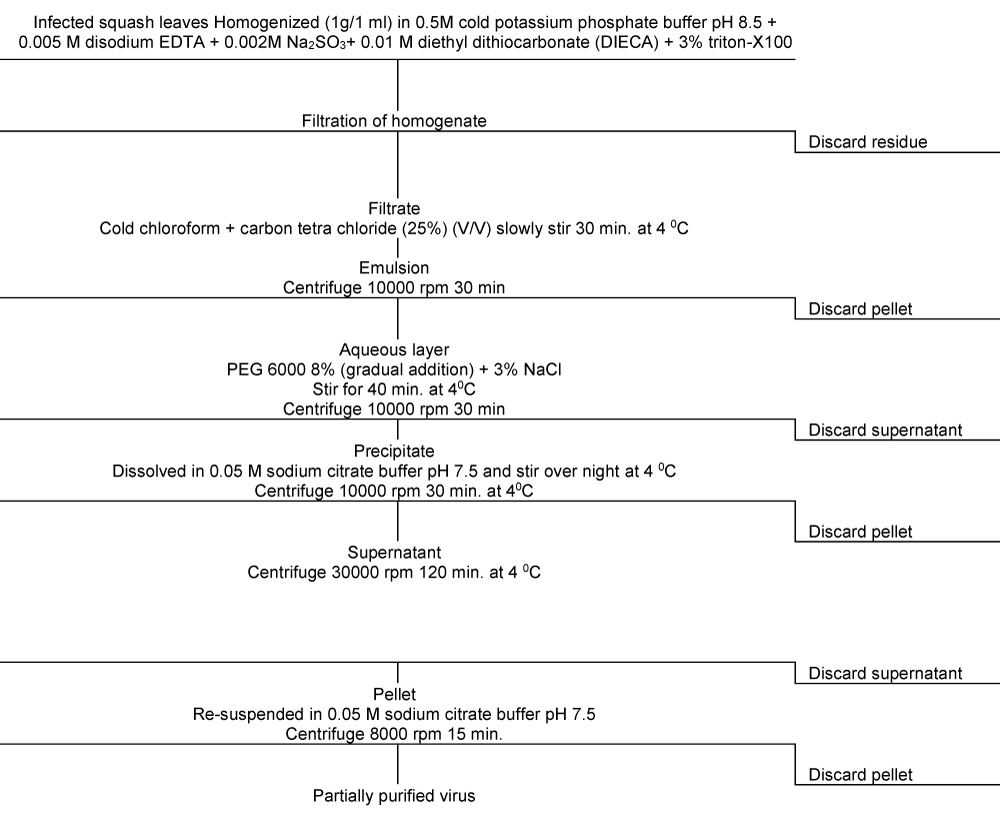
ZYMV was purified from systemically infected squash plants using the method described by Azzam and Makkouk [25] with some modifications. Two hundreds grams of infected leaf tissues harvested three weeks after inoculation were mixed with 200 ml of an extraction buffer [0.5 M K2HPO4, 0.002 M Na2SO3, 0.01 M Na-diethyldithiocarbamate (DIECA) and 0.01 M ethylenediamine-tetraacetate sodium salt (EDTA)] pH 8.5, and homogenized with a warring blender. The homogenate was filtered through 3 layers of cheesecloth and then 3% Triton X-100 was added (v/v). To the homogenate, a mixture of 25% chloroform and 25% carbon tetrachloride (v/v) was added slowly and stirred for 30 min. followed by low speed centrifugation (10000 rpm/30 min) at 4 °C. To the aqueous phase, 8% polyethyleneglycol (PEG), (mol. Wt. 6000) and 3% NaCl (w/v) were added and stirred for one hour at 4°C and subjected to 10000 rpm/30 min at 4 °C centrifugation using Beckman J-21 centrifuge and Type JA-14 rotor. The pellets were suspended in 0.05 M sodium citrate buffer, pH 7.5 and left overnight at 4 °C with occasional stirring. The resuspended pellet was centrifuged at 10000 rpm for 30 min at 4 °C. Further purification was obtained by a second precipitation with PEG, to the supernatant 8% PEG and 3% NaCl (w/v) were added and stirred for one hour at 4 °C and subjected to 30000 rpm/120 min centrifugation at 4 °C. The obtained pellets were resuspended in sodium citrate buffer and centrifuged at 8000 rpm/15 min (Figure 2). Virus presence was checked biologically by inoculating leaves of C. amaranticolor. U.V. absorption spectrum of the purified virus at a range of wave length 200-320 nm with 10 nm interval was recorded spectrophotometrically using Shimadzu UV-160 a spectrophotometer. A260/280 and A280/260 as well as virus concentration were estimated. The virus yield was calculated according to Noordam [26] as follows: Virus yield = O.D. (260) X Dilution factor/ E.C. Whereas: O.D. = optical density at 260 nm; E.C. = extinction coefficient 2.4 (mg/ml) [27].
Figure 2: Diagrammatic outline of ZYMV isolate purification by infected squash plant leaves.
Antiserum production for ZYMV
Antiserum to ZYMV was produced in New Zealand white rabbit by four weekly intramuscular injections with purified virus as Younes [14] with slid modifications. For each injection, virus concentration at 0.65 mg/ml was emulsified with an equal volume of Freund’s complete adjuvant in the first one and for subsequent injections with Freund’s incomplete adjuvant. The rabbit was bled 2 weeks after the fifth injection. The ZYMV antiserum obtained was stored in the presence of 0.05% sodium azide at -20 °C. The titer of ZYMV antiserum was determined by using indirect ELISA as described previously. Extracts from infected and healthy squash plants were diluted with coating buffer to 1:10. Serial dilutions of double fold up to 1:819200 of antiserum from cross- adsorption with filtered extracts from healthy tissues diluted 1:20 in serum buffer were used as [24].
Reactions of diagnostic hosts
Diagnostic hosts reacted with symptoms similar to those produced by ZYMV. The virus induced chlorotic local lesions without systemic spread on C. amaranticolor, (Figure 3d) and mosaic, yellowing, blistering and deformation on squash Eskandrani (Figure 3c). These symptoms are similar to those observed on naturally infected squash plants (Figure 3b). Squash c.v. Eskandrani showed severe yellow mosaic and malformation with blistering and shoe string battern and necrotic local lesion observed on inoculated cotyledons (Figure 3a). No symptoms were observed on G. globosa, Phaseolus vulgaris cv. Bountiful, Datura stramonium and N. glutinosa nor N. rustica.
Figure 3: a) necrotic local lesion observed on inoculated cotyledons of squash c.v. Eskandran; b) mosaic and yellowing on squash leaves; c) and mosaic, yellowing, blistering and deformation on squash Eskandrani; d) The virus induced chlorotic local lesions without systemic spread.
Serological reaction
ZYMV antiserum positively reacted with the isolated virus when used at a dilution of 1:500 in indirect ELISA. No positive reaction was detected with antisera to CMV and WMV-2 as determined by indirect ELISA (Table 1).
| Table 1: Reaction of infected squash fruits extract against antisera to CMV, WMV-2 and ZYMV as determined by indirect ELISA. | |||
| Squash fruits extract | Optical Density at 405 nm | ||
| CMV | WMV-2 | ZYMV | |
| I | 0.273 | 0.211 | 0.748 |
| H | 0.241 | 0.179 | 0.321 |
| H = Healthy; I = Infected | |||
Aphid transmission
The virus was transmitted none persistently with Aphis gossypii Glover, A. neri and Myzus persicae with average transmission rates of 60%, 70% and 90% respectively when ten viruliferous aphids were used on each test plant (Table 2).
| Table 2: Transmission of ZYMV with several Aphid sp. | ||
| Aphis species | Transmission | |
| Rate* | % | |
| Aphid gossypii | 6/10 | 60 |
| A. nerii | 7/10 | 70 |
| Myzus persicae | 9/10 | 90 |
| *Number of infected plants/no. of tested plants. 10 aphids were used per plant. | ||
Photometrical characters and yield of the purified virus
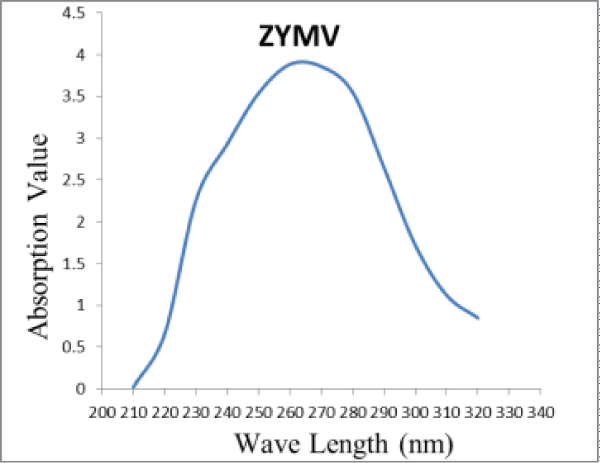
The Squash fruits isolate of ZYMV was purified by ultra-centrifugation and PEG. The absorption spectrum of the purified virus isolate determined through Jon Way 6405 UV/VIS spectrophotometer, was typical for nucleoprotein (Figure 4). The U.V. absorption spectrum of the purified virus preparation revealed typical spectrum of nucleoprotein. The ratios of A260/280 and A280/260 were 1.1 and 0.91 respectively. Concentration of the virus in the preparation was estimated using an extinction coefficient of E2600.1% = 2.4. The yield of the purified virus was about 1.62 mg/100 g fresh weight of squash leaves. Inoculum prepared from such purified virus induced numerous local lesions on inoculated leaves of C. amaranticolor were observed.
Figure 4: Ultra Violet absorption spectrum of ZYMV purified from Cucurbit pepo infected leaves.
Production of ZYMV antiserum
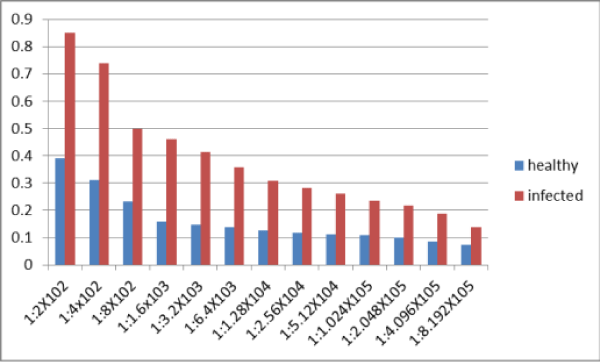
The antiserum against ZYMV was produced. Its titer was determined by indirect ELISA. Positive ELISA values were obtained up to dilutions of 1:409600 and not with 1:819200 (Figure 5).
Figure 5: Indirect ELISA Optical Density (E 405 nm) of extract of ZYMV infected squash plant leaves in various dilutions of ZYMV antiserum.* *(The experiment was repeated twice and ELISA optical density at 405 nm are average of two replicates each treatment. *Optical Density of at least double that of the healthy control were considered positive).
On the principle of symptoms developed on diagnostic hosts and serological reactions with antisera to CMV, WMV-2 and ZYMV were detected by indirect ELISA. The virus isolated from naturally infected Squash plants, grown in Abees Region, Alexandria governorate, with the symptoms of mosaic, yellowing, blisters and stunt symptoms, was identified as ZYMV. In Egypt, ZYMV was isolated from naturally infected squash plants [9-15]. ZYMV showed severe mosaic, yellowing, blistering and leaf deformation on most of cucurbits tested. ZYMV was readily sap transmissible to a limited range of hosts; these results are in harmony with the finding of Lisa and Lecoq and Purciful, et al. [1,28]. Gomphrena globosa reacted negativily with ZYMV isolated from squash, the same was found by Al-Shahwan and Younes, 2003 [7,14]. ZYMV is efficiently transmitted from plant to plant by aphids in a non-persistent manner [2]. The transmission efficiency varies between different aphid species [21] by Aphis gossypii [2,11,12,29]. The present study showed also that, in addition to Aphis gossypii, the squash isolate of ZYMV is transmited by Aphis neri and Myzus persicae. The purification of the isolated virus was carried out to determine the photometrical characters of the purified virus and to prepare specific antiserum. The yield of the virus as well as its specific photometrical data such as A260/280 and A 280/260 fall in the range reported for ZYMV [2,11,12,14,19,30,31], however, the slight differences in absorbance ratios as well as yield of the virus of ours and theirs may be attributed to the method used in purification as precipitation by PEG and ellipsis of final step for further purification such as ultracentrifugation or density gradient centrifugation [4,19,31,32]. Antiserum against the isolated ZYMV was produced and found to have titer of 1: 4096 X 105 as determined by indirect ELISA [14,33]. While, Bhargavi, et al. [31] was performed DAC-ELISA and the positive reaction up to 1/5000 titre of ZYMV antiserum was observed.
Squash is one of the important crops of cucurbitaceae grown in northern of Egypt. During field survey stunting of plants, yellow mosaic, blistering of the leaves and fruit deformation was observed. Both the natural symptoms on squash and artificial induced symptoms on squash seedlings were similar. In indirect ELISA with ZYMV antiserum it was reacted positively. Purification of ZYMV from infected squash leaves was purified by ultra-centrifugation with PEG. A good yield was gained from the infected leaves. A highly titer was observed by indirect ELISA.
The authors like to thank Proof. Dr. Hedaya Hamza, Entomology Department, Faculty of Agriculture, Alexandria University, Egypt. Mrs. Heba Awad for collecting the infected squash fruit samples from Abees, Alexandria, Egypt.
- Lisa V, Lecoq H. Zucchini yellow mosaic virus. CMI/AAB Description of Plant Viruses. 1984; No. 282.
- Lisa V, Boccardo G, D’Agostino G, Dellavalle G, d’Aquilo M. Characterization of a potyvirus that causes zucchini yellow mosaic. Phytopathology. 1981; 71:667-672.
- Lesemann DE, Makkouk KM, Koenig R, Natafji Samman E. Natural infection of cucumbers by zucchini yellow mosaic virus in Lebanon. Phytopathol Z. 1983; 108: 304-313.
- Davis RF. Partial characterization of zucchini yellow mosaic virus isolated from squash in Turkey. Plant Dis. 1986; 70:735-738.
- Al-Musa AM. Severe mosaic caused by zucchini yellow mosaic virus in cucurbit from Jordan. Plant Pathology. 1989; 38:541-546.
- Antignus Y, Raccah B, Gal-On A. Biological and serological characterization of zucchini yellow mosaic virus and watermelon mosaic virus-2 isolates in Israel. Phytoparasitica. 1989; 17: 289-297.
- Al-Shahwan IM. First report of zucchini yellow mosaic virus on cucurbits in the central region of Saudi Arabia. J. King Saud Univ. 1990; 2:251-260.
- Vučurović A1, Bulajić A1, Stanković I1, Ristić D1, Nikolić D1, et al. First Report of Zucchini yellow mosaic virus in Watermelon in Serbia. Plant Dis. 2012; 96: 149. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30731890
- Khalil EM, El-Said H, Attia MF, Ibrahim IA. Occurrence of zucchini yellow mosaic virus in Egypt. The 1st Nat. Conf. of Pest, Disease of Veg. and Field Crops. Ismailia, Egypt. 1985; 672-675.
- Ibrahim LM. Studies on some viruses affecting cucurbits in A.R.E. Ph. D. Thesis. Fac of Agric. Cairo Univ. Egypt. 1986; 119.
- Awad MAE, Masoad LI, Shalaby AA, Gamal El-Din AS. Characterization of zucchini yellow mosaic virus in Egypt. Egypt. J Applied Sci. 1994; 9: 1-9.
- Abdel-Ghaffar MH, El-Dougdoug KA, Sadik AS. Serology and partial characterization of the Egyptian isolate of zucchini yellow mosaic potyvirus. 1998; 6: 313-327.
- Farag AG. Studies on zucchini yellow mosaic virus in Egypt. 1999; 130.
- Younes HAA. Natural infection of luffa (Luffa aegyptiaca. Mill) with Zucchini Yellow Mosaic Virus in Egypt. J Adv Agric Res. 2003; 8: 227–239.
- Fath-Allah MM, Ahmed AA. Sensitive detection of watermelon mosaic and zucchini yellow mosaic viruses from infected squash plants using serological methods and polymerase chain reaction. Egyptian J Exper Biol. 2011; 7: 179-185.
- Hamza KA, Abd El-Aziz MH, Behiry SI, Younes HA. Isolation and purification of potato virus y isolate infecting potato (Solanum tuberosum l.) in al-nubaria region. Middle East J Agri Res. 2018; 7: 1201-1207 .
- Provvidenti R, Gonsalves D, Humaydan HS. Occurrence of zucchini yellow mosaic virus in cucurbits from Connenticut, New York, Florida, and California. Plant Dis. 1984; 68: 443-446.
- Huang CH, Liang SC, Deng TC, Hseu SH. Comparison of diagnostic hosts and serological tests for four cucurbit potyviruses. Plant Pathology Bulletin. 1993; 2: 169-176.
- Prieto, H., Bruna, A., Hinrichsen, P., and Muñoz, C. 2001. Isolation and molecular characterization of a Chilean isolate of Zucchini yellow mosaic virus. Plant Dis. 85:644-648.
- Usher L, Sivparsad B, Gubba A. Isolation, identification and molecular characterization of an isolate of Zucchini yellow mosaic virus occurring in KwaZulu-Natal, South Africa. South African J Plant Soil. 2012; 29: 65–71.
- Gal-On A. Zucchini yellow mosaic virus: insect transmission and pathogenicity—the tails of two proteins. Molecular Plant Pathology. 2007; 8: 139–150.
- Abd El-Aziz MH. Studies on some viruses infecting cowpea plants. 2019a; 165.
- Abd El-Aziz MH, Younes HA. Detection of Cucumber mosaic cucumovirus in infected cowpea plants (Vigna unguiculata L.) from northern Egypt. Novel Res Microbiol J. 2019; 3: 326 – 340 .
- Abd El-Aziz MH. Three modern serological methods to detect plant viruses. J Plant Sci Phytopathol. 2019b; 3: 101-106.
- Azzam OI, Makkouk KM. Purification of two potyvirus isolates infecting Phaseolus vulgaris L. in Lebanon. Phytopathological Mediterranean. 1986; 25: 125-130.
- Noordam D. Identification of Plant Viruses Methods and Experiments". Centre for Agricultural Publishing and Documentation, Wageningen, the Netherlands. 1973; 207.
- Abd El-Aziz MH. The Importance of Potato virus Y Potyvirus. J Plant Sci Phytopathol. 2020; 4: 009-015.
- Purcifull DE, Adlerz WC, Simone GW, Hiebert E, Christie SR. Serological relationships and patial characterization of zucchini yellow mosaic virus isolated from squash in Florida. Plant Dis. 1984; 68:230-233.
- Mahgoub HA, Desbiez C, Wipf-Scheibel C, Dafalla G, Lecoq H. Characterization and occurrence of zucchini yellow mosaic virus in Sudan. Plant Pathol. 1997; 46: 800-805.
- Greber RS, Persley DM, Herrington ME. Some characteristics of Australian isolates of zucchini yellow mosaic virus. Aust J Agric Res. 1988; 39:1085-1094.
- Bhargavi V, Gaddam SA, Kotakadi VS, Sai Gopal DVR. Partial Characterization of Zucchini yellow mosaic virus infecting Gherkin (Cucumis anguira L.) in chittoor district of ndhra Pradesh, India. Res J Pharmaceutical Biological Chem Sci. 2017; 8: 522-526.
- Huang CH, Hseu SH. Purification, serology and properties of five zucchini yellow mosaic virus isolates. Plant Pathol. 1989; 38: 414-420.
- Fegla GI, El-Samra IA, Younes HA, Abd El-Aziz MH. Comparative studies for detection of Tomato mosaic tobamovirus (ToMV), cucumber mosaic cucumovirus (CMV) and Potato Y potyviruses (PVY). Adv Agric Res. 2001; 6: 239–254.