More Information
Submitted: 13 September 2019 | Approved: 30 September 2019 | Published: 01 October 2019
How to cite this article: Abd El-Aziz MH, Younes HA. Serological and molecular characterization of two seed born cowpea mosaic Comovirus isolates affecting cowpea plants (Vigna unguiculata L.) in northern Egypt. J Plant Sci Phytopathol. 2019; 3: 086-094.
DOI: 10.29328/journal.jpsp.1001037
Copyright License: © 2019 Abd El-Aziz MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Serological and molecular characterization of two seed born cowpea mosaic Comovirus isolates affecting cowpea plants (Vigna unguiculata L.) in northern Egypt
Mahmoud Hamdy Abd El-Aziz1* and Hosny Aly Younes2
1Plant Pathology Institute, Agricultural. Research Center, Alexandria, Egypt
2Agricultural Botany Department, Faculty of Agriculture, Saba Basha, Alexandria University, Egypt
*Address for Correspondence: Mahmoud Hamdy Abd El-Aziz, Plant Pathology Institute, Agricultural. Research Center, Alexandria, Egypt, Tel: +201229556042; Email: [email protected]; [email protected]
Cowpea plants naturally infected with cowpea mosaic comovirus (CPMV) showed different mosaic, mottle, dwarfing, and vain clearing symptoms. Diseased plants were ollected from certain locations of Alexandria and El-Beheira governorates during the growing seasons from 2011 to 2012. CPMV was detected in infected sap at 8 to 24 days after inoculation by DBIA, indirect ELISA and tissue blot immunoassay (TBIA). Chlorotic local lesions were observed on Chenopodium amaranticolor in infectivity test. By using indirect ELISA and DBIA, CPMV were detected in infected plant sap of serial dilutions up to 1: 400. The incidence of CPMV in 21 day old cowpea seedlings grown from infected seeds was determined by ELISA and positive detection of virus antigen reached 65%. Nitrocellulose membrane and canson paper could be used as solid carriers in TBIA and DBIA for detection of CPMV in infected plant tissues. Results revealed that both faces of nitrocellulose membrane and canson paper could be used as solid carriers in TBIA for detection of CPMV in infected plant tissues. According to reverse transcription polymerase chain reaction (RT-PCR) assay of CPMV infected plant; the amplified product was approximately 800bp of partial coat protein gene. The nucleotide sequences accession number were LN606585 and LN606586. The phylogenetic tree was generated using sequences of CPMV isolates with the other CPMV records from GenBank.
Cowpea (Vigna unguiculata L.) has played an important role as a feeding crop. It can be used as green or dried seeds as a good diet for its high protein content [1]. In Egypt, under field conditions cowpea is subjected to infection with cowpea mosaic comovirus (CPMV) [1,2]. CPMV is the type member of the Genus Comovirus in the family Comoviridae.
Bruening and Agrawal, [3]. Little work has been done on Cowpea mosaic comovirus (CPMV) in Egypt. It is one of the most commonly reported virus diseases of cowpea, which causes mosaic and decreases leaf area and flower production [4]. Symptoms induced by CPMV vary from light green mottle to distinct yellow mosaic, distortion of leaf, and premature death of the plant [5]. In our research detection of CPMV infecting cowpea in northern Egypt was based mainly on serological diagnosis and RT-PCR. The aims of this work were detection and isolation of CPMV affecting cowpea plants. Viral characterization included host range and symptomology, modes of transmission, sap infectivity tests, serological tests and nucleotide sequencing. Detection of CPMV isolate1 in various plant organs such as floral parts, pods, seed and seed parts showed positive results. Comparative studies for detection of CPMV by TBIA, DBIA, indirect ELISA and biological assay in infected plants were conducted after different periods of inoculation, and in serial dilutions of infected plant sap. Possibility of using alternative solid carriers instead of nitrocellulose membranes in TBIA and DBIA tests was determined using two faces of the solid carriers in TBIA test. Partial sequences of the virus isolates was registered in gene bank.
Leaf samples of Cowpea (Vigna unguiculata) showing mosaic, mottle, dwarfing, and vein clearing symptoms were separately collected in plastic bags from naturally infected cowpea plants grown at certain locations of Alexandria and El-Beheira governorates during the growing seasons from 2011 to 2012. Virus inoculum was prepared by grinding infected leaf tissues 1:10 (W/V) with a mortar and pestle in 0.1M phosphate buffer, pH 7.0, containing 0.5% 2-mercaptoethanol. Leaves of healthy plants in seedling stage were first dusted with carborundum (600 mesh) and then inoculated with a freshly prepared inoculum using forefinger method and kept in insect proof greenhouse under observation.
Diagnostic hosts and symptomology
The diagnostic hosts as Chenopodium amaranticolor, Datura stramonium, Glycine max, Nicotiana rustica, N. glutinosa, Phaseolus vulgaris and Vigna unguiculata were used to observe characteristic symptoms for identification of the isolated virus. Five seedlings of each tested plant species or cultivar were mechanically inoculated with CPMV isolates and kept under greenhouse conditions. Plants were examined daily, for four weeks for symptoms expression. Inoculated plants that did not show any symptoms were cheeked for latent infection by back-inoculation to the indicator host C. amaranticolor [6-10].
Serological diagnosis
Serological diagnosis was carried out using indirect ELISA.
Source of antisera: Antiserum of cowpea mosaic comovirus (CPMV) used in this study was kindly supplied by Younes, et al. [10]. Antisera of cucumber mosaic cucumovirus (CMV), alfalfa mosaic alfamovirus (AMV), cowpea aphid borne mosaic potyvirus (CABMC) and Tobacco ringspot nepovirus (TRSV) used in this study were kindly supplied by Antiserum –Bank, Institute of seed pathology for Developing Countries, Denmark.
Indirect ELISA: Indirect ELISA was carried out as described by Hamza, et al. [11]. Extracts from infected and healthy cowpea plants were detected. The ELISA values, measured by Sunrise ELISA plat reader, were expressed as absorbance at 405 nm. Absorbance valued of at least double that of healthy control, were considered positive.
In each set of test, wells lacking antigen (coating buffer only) were included as blanks.
Transmissibility of CPMV
Mechanical transmission: Cowpea c.v. Kareem7 plants were infected for use as a virus source, whereas, Chenopodium amaranticolor was an assay host. The infected cowpea leaves showing typical disease symptoms were ground 1:10 (w/v) using mortar and pestle in 0.1M phosphate buffer (pH 7.0), containing 0.5% 2-mercaptoethanol. Leaves of healthy Vigna unguiculata plants were first lightly dusted with carborandum (600 mesh), and then rubbed with forefinger previously soaked in the freshly prepared inoculum according to Hamza, et al. [11].
Seed transmission: Seeds of cowpea c.v. Kareem7 obtained from Agricultural Research Center, Legumes Research Department, Ministry of Agriculture, Giza were sown according to Abd El-Aziz, [1]. Seedlings were separately inoculated with CPMV isolate1, three weeks after sowing. CPMV isolate1 was detected in seedlings using indirect ELISA as [1,10].
Serological detections
Detection of CPMV in flower and floral parts: Cowpea plants infected with CPMV isolate1 in primary leaf stage and recurred till flowering. The flower was divided to its parts and then collected in groups each group contain 5 replicates. Groups of five flowers, calyx, corolla, androecium and gynoecium were diagnosed for CPMV with DBIA and indirect ELISA.
Serological detection by Dot blot immunoassay (DBIA): DBIA of Powell [12], optimized by Fegla, et al. [13] with slid modifications was used. A grid consisting of 1 cm squares was drawn on nitrocellulose membrane sheet (NCM 0.45 nm, BIO-Rad Laboratories, Richmond, CA) with pencil. The sheet was then cut to a size that would accommodate the number of samples in an individual test. The tested samples were ground with a mortar and pestle in carbonate buffer (0.05 M carbonate pH 9.6) 1:10 (w/v) and strained by 4 layers of cheesecloth then centrifuged at 8000 rpm for 20 min. The nitrocellulose membrane was dipped in 0.05 carbonate buffer pH 9.6 and placed on filter paper for 5 min. to dry. Two micro-liter of each sample was spotted on the nitrocellulose membrane in the center of each grid square and dried for 5 min. the membrane was then placed in a Petri dish containing 10 ml blocking solution (2% bovine serum albumin in carbonate buffer pH 9.6) and gently agitated for one hour (40 oscillations per minute). The membrane was removed from the blocking solution, dipped in distilled water and transferred to another Petri dish containing 10 ml of virus antiserum (1:500 diluted in PBST), the solution contained clarified plant sap (1 g healthy plant leaf tissue per 20 ml of PBST buffer triturated and clarified by centrifugation at 8000 rpm for 20 min.) and gently agitated for 2 hours. The membrane was removed from the first antibody solution, dipped in distilled water, and washed twice by agitation for 10 min. in phosphate buffer + tween 20, and transferred to 1:1000 dilution of goat anti-rabbit IgG conjugate to alkaline phosphatase in PBST and gently agitated for one hour. Finally, the membrane was removed from the second antibody dilution, dipped in distilled water and washed twice by agitation for 10 min. each in phosphate buffer + tween 20 (PBST). The 5- bromo- 4- chloro- 3- indolyl 1 phosphate (BCIP) and nitro blue tetrazolium (NBT) substrate solution was made during the final washing in which membrane was incubated for color development. After color development the reaction was stopped by washing the treated membrane in 0.01 M phosphate buffer containing 0.05 M EDTA pH 7.0. The positive reaction of DBIA was indicated by the development of purple color on the blots and the negative reaction development no more or green color.
Detection of CPMVisolate1 in pod and seed parts: Cowpea plants c.v. Kareem7 infected with CPMV in primary leaf stage and recurred till flowering and fruit stage. Mature green pods after 21 days of pod setting were collected from healthy and CPMVisolate1 artificially infected plants, using extraction ratio 1:10 (w/v). The pod was divided to its parts and then collected in groups each group contain 5 replicates of pods, testa, cotyledons and embryo and were diagnosed for CPMV with DBIA and indirect ELISA as previously described. Firstly, printed the green matured pods on NCM sheet to detect by TBIA.
Detection of CPMV in infected plants after different periods of inoculation: The extracts of CPMV infected cowpea leaves diluted 1:10 after different periods of inoculation for 1, 2, 3, 4, 5, 6, 8, and 10 days were compared for detection by TBIA, DBIA, indirect ELISA and infectivity test. CPMV antiserum was used at dilution of 1:500 in serological tests. Extracts of healthy cowpea leaves were served as control [14].
Serological detection by tissue blot immunoassay (TBIA): Samples of apparently infected and healthy seedlings from seeds of CPMV isolate1 infected plants cowpea c.v. Kareem7 were checked serologically for virus presence by tissue blot immunoassay (TBIA). With slid modifications TBIA as described by Lin, et al. [15] and modified by Fegla, et al. [16] was used. Tissue of rolled leaves; stems and roots from healthy and infected plants were cut with razor blades. Exposed cut edges were pressed on nitrocellulose membrane (NCM 0.45 nm, BIO-Rod Laboratories, Richmond, CA), previously dipped in 0.05M carbonate buffer (pH 9.6), and then placed on filter paper for 5 min. to dry. Treated membranes were then placed in a glass Petri plates containing 10 ml of blocking buffer (2% Bovine serum albumin (BSA) in phosphate buffer saline (PBST), pH 7.0), gently agitated for 1h. The membrane was removed from the blocking solution; dipped in dist. water, and then transferred to a virus antiserum (CPMV was diluted to 1:500 in PBST). The membrane was gently agitated for 2 h. removed from the first antibody solution, dipped in dist. water, and then washed twice by agitation for 10 min. in phosphate buffer + Tween-20 (PBST). The membrane was dipped in dist. water; transferred to a 1:1000 dilution of goat antirabbit IgG conjugated to alkaline phosphatase in PBST, and then gently agitated for 1h. Finally, the membrane was removed from the second antibody dilution, dipped in distilled water and washed twice by agitation for 10 min. each in phosphate buffer + tween 20 (PBST), and the rest of the procedure was followed as previously mentioned with DBIA. The positive reaction of TBIA was indicated by the development of purple color on the blots. The negative reaction developed no color or green color.
Detection of CPMV in serial dilutions of infected plant sap: The infected samples were collected 15 days after inoculation with each virus. Serial dilutions of cowpea leaves infected with CPMV beginning from 10-1 to 10-4 were tested with indirect ELISA, and DBIA and infectivity test. CPMV antiserum was diluted 1:500 in serological reactions as [17].
Possibilities of reducing the cost of TBIA and DBIA by using alternative solid carriers instead of nitrocellulose membrane: Comparison between canson paper (150 g/m2) was chosen as a regular type of paper by Abd El-Aziz and Youns, [14], and Abd El-Aziz, [18]. with nitrocellulose membrane (NCM, 0.2 nm and 0.45 nm Bio-Rad Laboratories, Richmond, CA) were carried out to use in TBIA and DBIA.
Possibility of using two faces of the solid carrier: Nitrocellulose membrane and canson paper were chosen to study the possibility of using both sides for virus detection by TBIA, [14]. Both faces of nitrocellulose membrane and canson paper were printed by healthy and CPMV infected cowpea leaves and stems, then the procedures of TBIA was followed as previously described.
Biological assay (Infectivity test)
Chenopodium amaranticolor at 4-5 leaf stage was used as a local lesion assay hosts for CPMV. It was mechanically inoculated with serial dilutions of infected cowpea leaf extract with CPMV beginning from 10QIAGEN 1-Step RT-PCR kit (Cat No. /ID: 210210; for highly sensitive and specific one-step RT-PCR). The cDNA synthesis was done using the following: 50 °C for 15 min. at 1 cycle to allow reverse transcription to be performed, verso inactivation at 95 °C for 2 min (1 cycle). The RT-PCR reaction followed by: 35 cycles to 10-4 as described above and kept in insect proof greenhouse under observation [1].
Molecular detection of CPMV by RT-PCR
The specific primers of CPMV was synthesized by Bio Basic Inc., Canada. The sequences of the forward and reverse primers (5′TGGCTGATTGTCAGAATTGG3′) and (5′ TTGACCATCCCAATCTGCTC3′), respectively, were used for detection of the CPMV S coat protein gene according to Phelps, et al. [19]. The cDNA synthesis of the CPMV-CP gene was based on the method given in the Thermo Scientific Verso at 95 °C for 1 min, 55 °C for 45 sec. and 72 °C for 1 min followed by 7 min incubation at 72 °C using a Hybaid thermal reactor [1].
Sequence analysis of CPMV-CP gene and phylogenetic tree: Coat protein gene of CPMV were sequenced using Chromas Pro Version 1.34 software, all isolates were compared with previously described sequences in the NCBI database with the program BLAST. Pairwise and multiple DNA sequence alignment were carried out using CLUSTALW multiple sequence alignment programmer version 1.82 (http://www.ebi.ac.uk/clustalw), [20]. Bootstrap neighbor joining tree was generated using MEGA version 3.1 [21] from CLUSTALW alignment. Comparison with sequences in the Gene Bank. Database was achieved in BLASTN searches at the National Centre for Biotechnology Information site (http://ncbi.nlm.nih.gov).
___________________________________________________________________________10-1 to 10-4 as described above and kept in insect proof greenhouse under observation [1].
Serological detection
Serological detection revealed the involvement of virus namely cowpea mosaic comovirus (CPMV) with symptoms of collected samples (Table 1). CPMV which used in this study was isolated from naturally infected seed of cowpea plants collected from different location of Alexandria and El-Behera Governorates, showing mosaic, severe mosaic, mottling and leaf deformation with vein clearing on cowpea leaves. CPMV isolated in this study caused mosaic, vein clearing, blisters and deformation on cowpea (Vigna unguiculata) c.v. Kareem 7 leaves. The natural infection CPMV isolate1 caused mosaic, crinkle and malformation on
| Table 1: Detection of some viruses infecting cowpea in leaf samples by Indirect ELISA optical density at 405 nm. | |||||
| Indirect ELISA absorbance values | |||||
| No. of represented tested samples | (E 405 nm) | ||||
| CPMV | CMV | AMV | CABMV | TRSpV | |
| 1 | 0.261 | 0.286 | 0.229 | 0.199 | 0.208 |
| 2 | 0.580 | 0.331 | 0.209 | 0.227 | 0.201 |
| 3 | 0.264 | 0.302 | 0.215 | 0.173 | 0.184 |
| 4 | 0.340 | 0.297 | 0.180 | 0.186 | 0.213 |
| 5 | 0.614 | 0.319 | 0.254 | 0.193 | 0.167 |
| 6 | 0.269 | 0.295 | 0.260 | 0.212 | 0.181 |
| H | 0.232 | 0.280 | 0.196 | 0.145 | 0.153 |
| The experiment was repeated twice and ELISA absorbance values at 405 nm are average of two replicates each. Absorbance values of at least double that of the healthy control were considered positive. H = Healthy, Bold = Positive reaction. | |||||
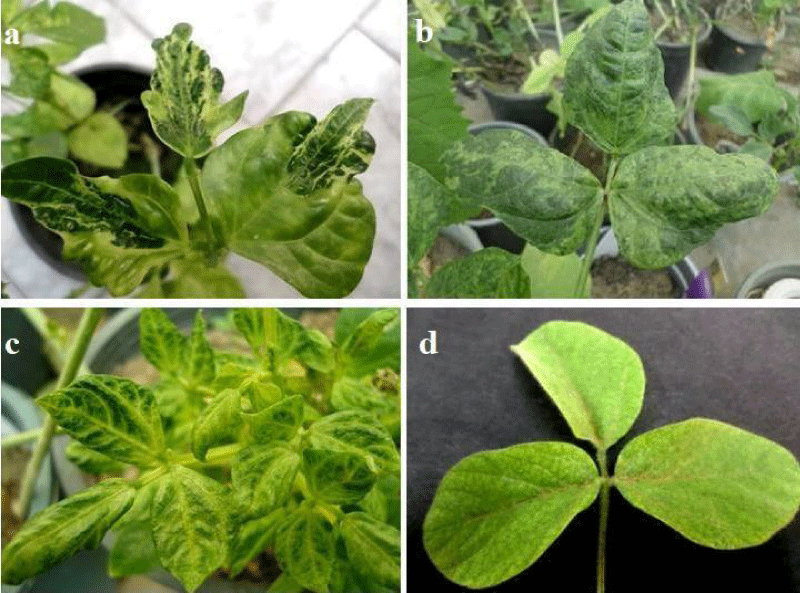
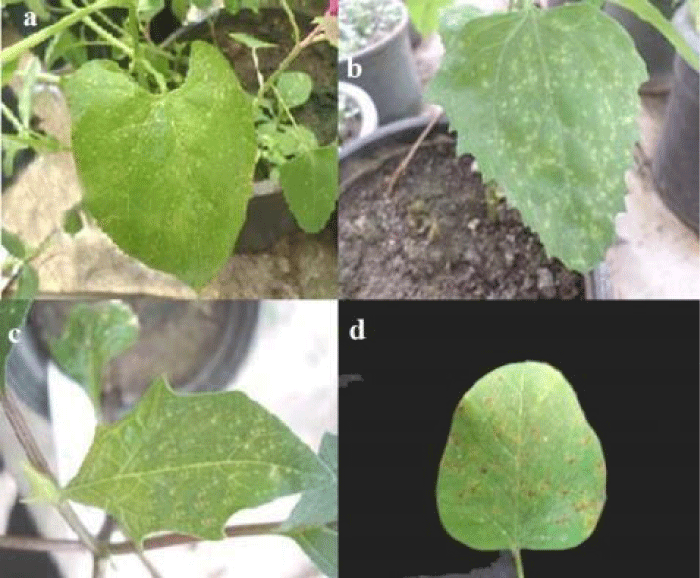
Vigna unguiculata cv. Red. while the natural infection CPMV isolate2 caused vein clearing, mosaic and blisters on V. unguiculata cv. Kareem7 leaves. Symptoms caused by CPMV isolate1 on inoculated leaves of Glycine max was mild mosaic, while on Phaseolus vulgaris and Datura stramonium was necrotic local lesions (Figure 2a,c). Symptom on inoculated leaf of Chenopodiuam amaranticolor was chlorotic local lesions (Figure 2b), and on inoculated primary leaf of Glycine max was necrotic spots (Figure 2d).
Figure 1: a) Symptoms caused by CPMV (isolate 1) on cowpea (Vigna unguculata) c.v. Kareem7 leaves showing mosaic, vein clearing, blisters and deformation, b) Natural infection caused by CPMV (isolate 1) on cowpea Vigna unguculata c.v. Red cowpea leaves showing mosaic, crinkle and malformation, c) Natural infection caused by CPMV (isolate 2) on cowpea (Vigna unguculata c.v. Kareem7 leaves showing vein clearing, mosaic and blisters, d) Symptoms caused by CPMV isolate 1 on Glycine max leaf showing mild mosaic.
Figure 2: a) Necrotic local lesions induced by CPMVisolate1 on Phaseolus vulgaris, inoculated leaf, b) Chlorotic local lesions caused by CPMVisolate1 on Chenopodiuam amaranticolor, inoculated leaf, c) Necrotic local lesions caused by CPMVisolate1on Datura stramonium, inoculated leaf, d) Necrotic spots caused by CPMVisolate1 on inoculated primary leaf of Glycine max.
Mode of transmission
Mechanical transmission: CPMV isolates were easily transmitted mechanically using 0.1 M phosphate buffer, pH 7.0 on cowpea (Vigna unguculata) plants with 100% infection.
Seed transmission: Detection of CPMV in a seedling stage: The incidence of CPMV was determined in 21 day old cowpea seedlings grown from seeds collected from infected cowpea plants for detected with indirect ELISA. Results are presented in table 2. Data concerning detection of virus antigen in 21 day old seedlings were presented in table 2. Infection percentage was 65% for testing 21 day old seedlings with ELISA.
| Table 2: Incidence of CPMV in 21 day old seedlings of cowpea cv. Kareem7 detected by ELISA. | |
| Group NO. | Indexing values of infected seedlings |
| **ELISA | |
| Control | 0.350 |
| 1 | 0.799 |
| 2 | 0.949 |
| 3 | 0.851 |
| 4 | 0.748 |
| 5 | 0.755 |
| 6 | 0.689 |
| 7 | 0.805 |
| 8 | 0.831 |
| 9 | 0.899 |
| 10 | 0.645 |
| 11 | 0.572 |
| 12 | 0.441 |
| 13 | 0.417 |
| 14 | 0.709 |
| 15 | 0.746 |
| 16 | 1.215 |
| 17 | 0.605 |
| 18 | 0.484 |
| 19 | 0.786 |
| 20 | 0.803 |
| Total % | 65% |
| *No. of infected seedlings out of 5 tested. **Extracts of groups each of 5 were used for ELISA. |
|
Serological Comparative studies for detection of the isolated CPMV
Collected samples for testing were first used for TBIA, subsequently, required preparations were done for DBIA and indirect ELISA.
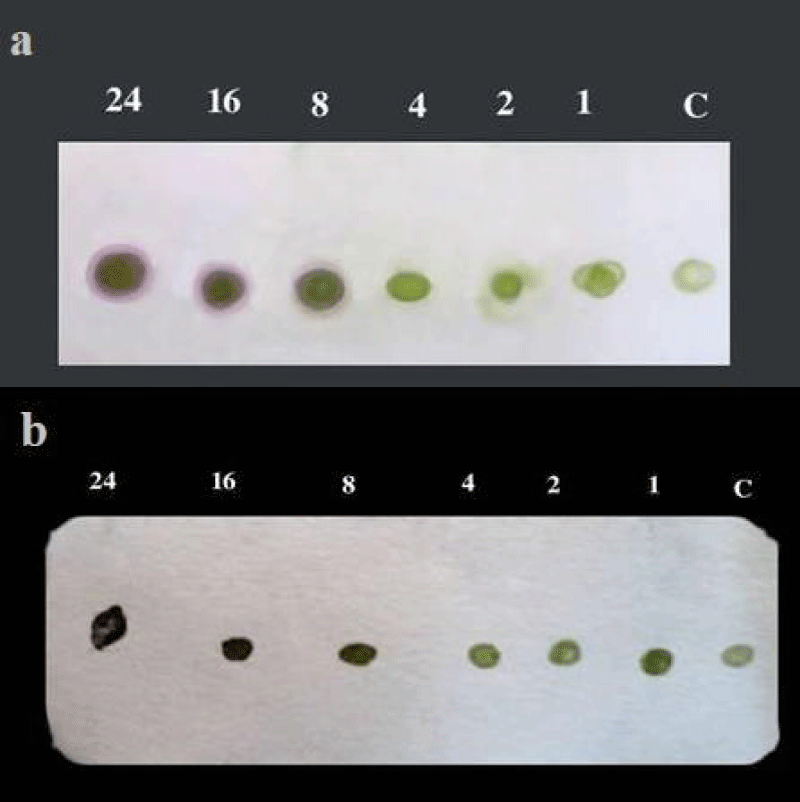
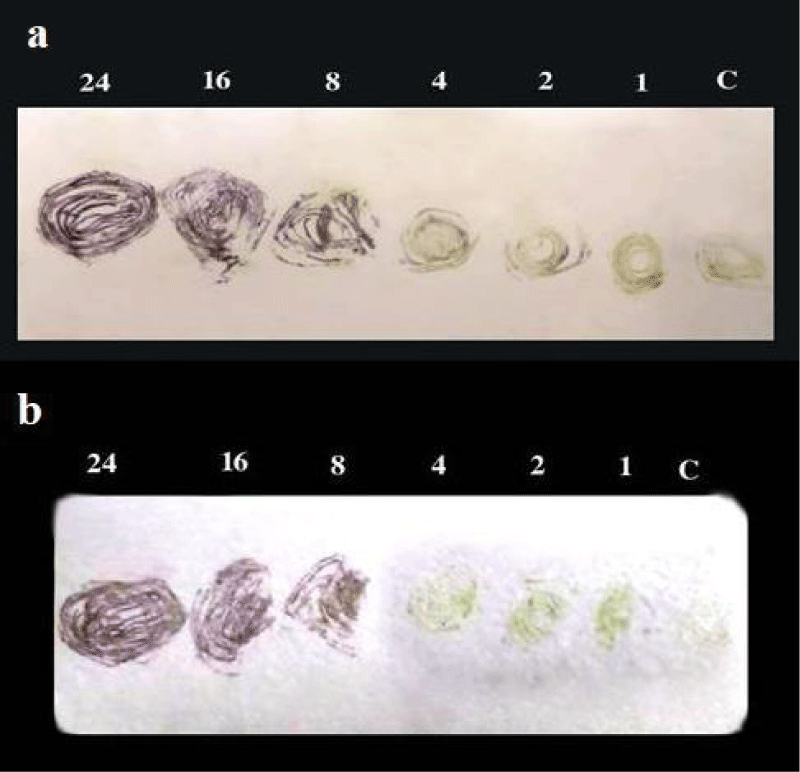
Detection in infected plants after different periods of inoculation: TBIA, DBIA and Indirect ELISA were used to detect the isolated CPMV (isolate1) in infected cowpea plants after 1, 2, 4, 8, 16 and 24 days of mechanical inoculation were tested. Obtained results showed that CPMV antiserum 1:500 could detect the virus in infected sap at 8, 16 and 24 days after inoculation by indirect ELISA (Table 3) and 8, 16 and 24 days after inoculation by DBIA on nitrocellulose membrane (Figure 3a) and on canson paper (Figure 3b) and so that 8, 16 and 24 days after inoculation by TBIA on nitrocellulose membrane (Figure 4a) and canson paper (Figure 4b).
| Table 3: Indirect ELISA for CPMVisolate1 in 1:10 dilution of sap extracted from infected plants at different periods after inoculation. | |
| Days | Indirect ELISA absorbance values (E 405 nm) |
| 24 | 0.454 |
| 16 | 0.394 |
| 8 | 0.357 |
| 4 | 0.291 |
| 2 | 0.214 |
| 1 | 0.203 |
| Healthy | 0.126 |
Figure 3: a) Sensitivity of DBIA on NCM for detection of CPMVisolate1 in infected cowpea plants after different periods (1-24 days) of mechanical inoculation. b) Sensitivity of DBIA on canson paper for detection of CPMVisolate1 in infected cowpea plants after different periods (1-24 days) of mechanical inoculation.
Figure 4: a) Sensitivity of TBIA on NCM for detection of CPMVisolate1 in infected cowpea plants after different periods (1-24 days) of mechanical inoculation. b) Sensitivity of TBIA on canson paper for detection of CPMVisolate1 in infected cowpea plants after different periods (1-24 days) of mechanical inoculation.
Detection of CPMV in serial dilutions of infected plant sap: Indirect ELISA was used to detect the isolated virus CPMV in infected plant sap in serial dilutions of 1:10, 1:50, 1:100, 1:200 and 1:400 in 0.05M carbonate buffer pH 9.6 from infected plant leaves obtained by mechanical inoculation. Obtained results showed that CPMV isolate1 antiserum 1:500 could detect the virus in infected sap at dilution 1:400 by indirect ELISA (Table 4).
| Table 4: Indirect ELISA for CPMVisolate1 in serial dilutions of infected cowpea plant sap. | ||
| Dilutions | Infected | Healthy |
| 1:10 | 0.524 | 0.241 |
| 1:50 | 0.448 | 0.185 |
| 1:100 | 0.382 | 0.137 |
| 1:200 | 0.336 | 0.146 |
| 1:400 | 0.319 | 0.129 |
Infectivity tests
Serial dilutions of mechanically inoculated cowpea plants with CPMV isolate1 such as 1:10, 1:50, 1:100, 1:200 and 1:400 in 0.1 M phosphate buffer pH 7.0 and mechanically inoculate assay local lesion host (Chenopodium amaranticolor). Chlorotic local lesions were observed up to 1: 400 dilutions as table 5.
| Table 5: Dilution end point of CPMVisolate1 infectivity test on Chenopodium amaranticolor. | |
| Dilutions of infected plant sap | Mean* of observed CLL |
| 1:10 | 151 |
| 1:50 | 137 |
| 1:100 | 119 |
| 1:200 | 69 |
| 1:400 | 27 |
| *each treatment containing 10 Chenopodium amaranticolor leaves and replicated two times. | |
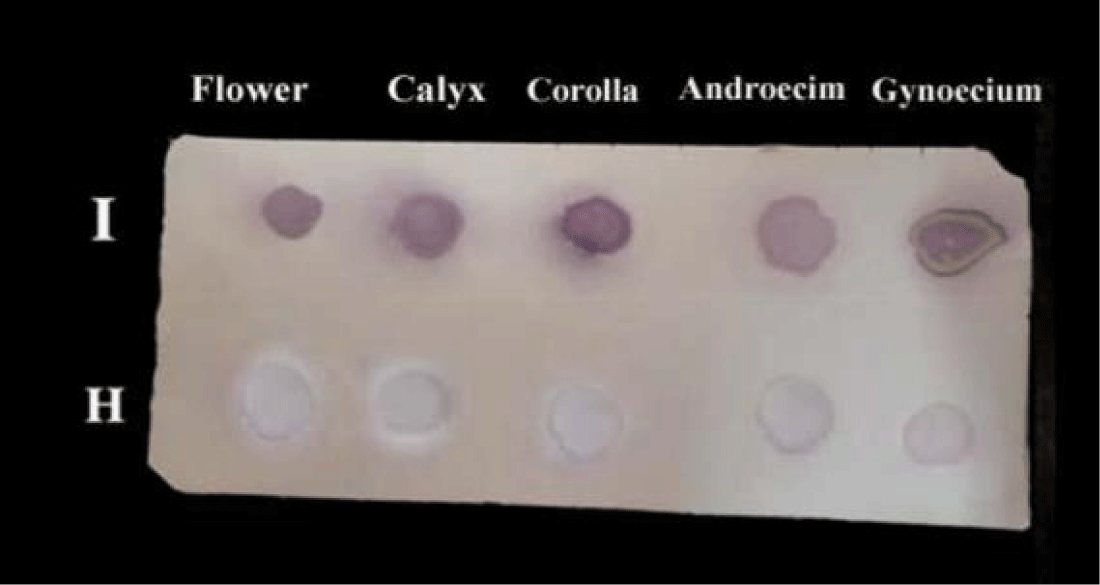
Detection of CPMV in floral parts: Cowpea plants infected with CPMVisolate1 in primary leaf stage grown till flowering and fruit stage. The results (Table 6 and Figure 5) shows the sensitivity of DBIA on nitrocellulose membrane and the ability for detection the CPMV isolate1 in infected floral parts.
| Table 6: Indirect ELISA for CPMV in 1:10 dilution of sap extracted from infected flowers and floral parts of cowpea plants. | ||
| Floral parts | Infected | Healthy |
| Flowers | 1.35 | 0.62 |
| Calyx | 1.27 | 0.457 |
| Corolla | 1.18 | 0.54 |
| Androecim | 1.18 | 0.57 |
| Gynoecium | 1.34 | 0.82 |
Figure 5: Sensitivity of DBIA on nitrocellulose membrane for detection of CPMVisolate1 in infected flower and floral parts of cowpea cv. Kareem7 after mechanical inoculation. I = infected; H = healthy,
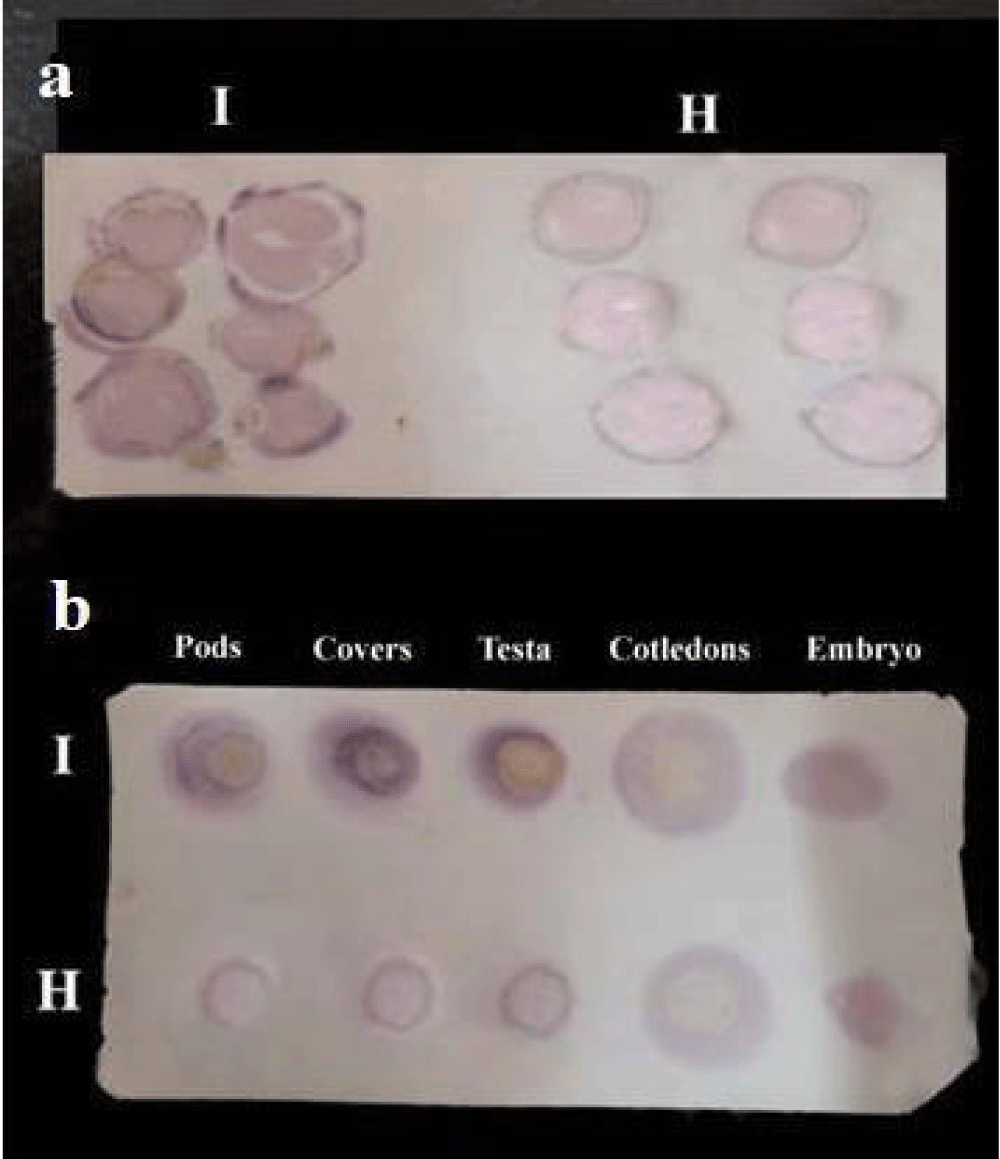
Figure 6: a) Sensitivity of TBIA on nitrocellulose membrane for detection of CPMVisolate1 in infected pods of cowpea c.v. Kareem7 after mechanical inoculation. b) Sensitivity of DBIA on nitrocellulose membrane for detection of CPMVisolate1in infected pods and seed parts of cowpea c.v. Kareem7 after mechanical inoculation. I = infected; H = healthy.
Detection of CPMV in green pods and immature seed parts: The pods and seed parts of the diseased pods were shown in table 7 and figure 6a shows the results described the sensitivity of TBIA on nitrocellulose membrane for detection of CPMV isolate1 in infected pods of cowpea cv. Kareem7 after mechanical inoculation. Figure 6b shows the results of diseased immature seed parts.
| Table 7: Indirect ELISA for CPMV in 1:10 dilution of sap extracted from infected pods and seed parts of cowpea plants | ||
| Pods and seed parts | Infected | Healthy |
| Pods | 0.77 | 0.26 |
| Covers | 0.96 | 0.46 |
| Tasta | 0.49 | 0.24 |
| Cotyledons | 0.45 | 0.29 |
| Embryo | 0.35 | 0.29 |
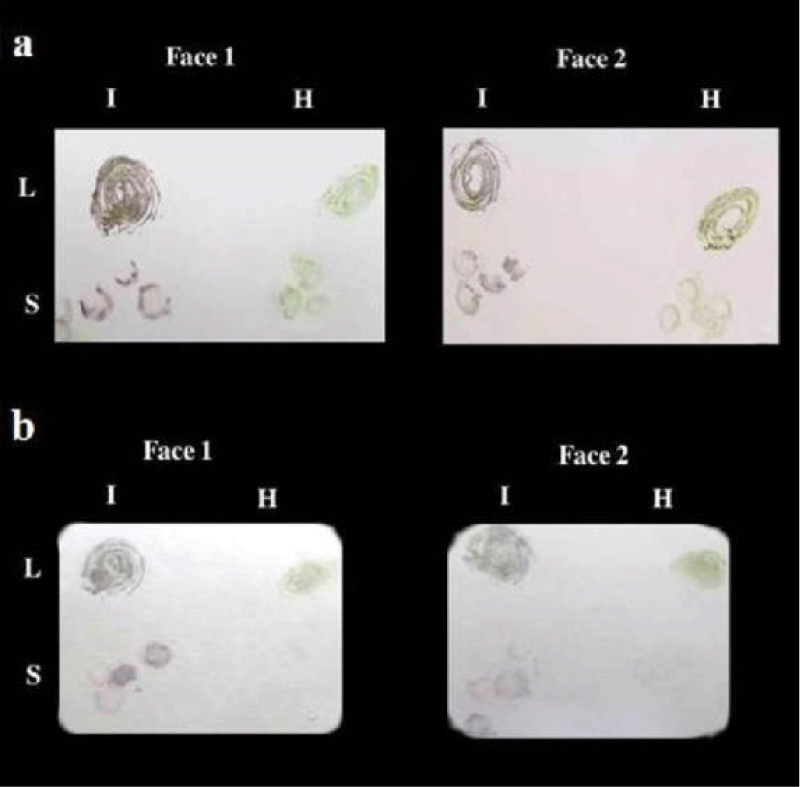
Possibilities of reducing the cost of TBIA by using two faces of the solid carrier: Results showed that both faces of nitrocellulose membrane and canson paper could be used as solid carriers in TBIA for detection of CPMV (Figure 7a,b). Since positive reactions of both tests on both faces of solid carriers were clearly observed.
Figure 7: a) TBIA used for detection of CPMVisolate1 in infected leaves and stems of cowpea plants on both faces at the same time on Nitrocellulose membrane. b) TBIA used for detection of CPMVisolate1 in infected leaves and stems of cowpea plants on both faces at the same time on canson paper. L = leaves; S = stems I = infected; H = healthy.
Detection by reverse transcription polymerase chain reaction (RT-PCR)
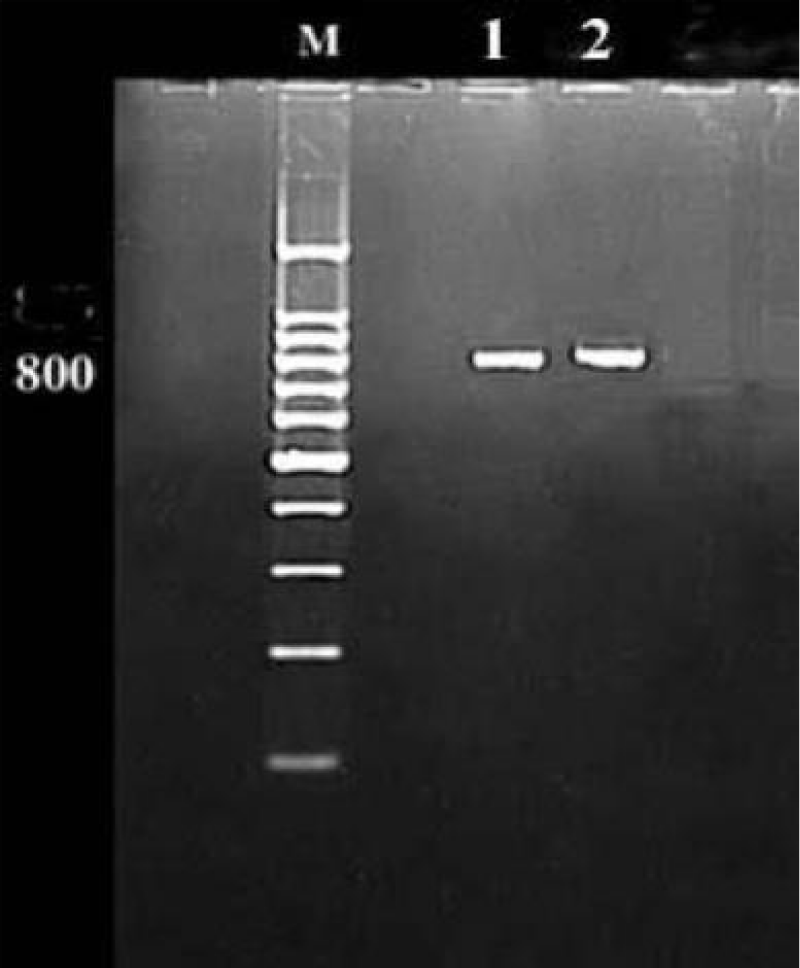
Reverse transcription polymerase chain reaction (RT-PCR): was performed on total RNA extracted from infected cowpea leaf tissues showing infection with the two virus isolates. The obtained results confirmed the serological diagnosis and the specificity of the primers used in this study. The size of amplification obtained product was approximately 800 bp for both isolates of CPMV (Figure 8).
Figure 8: Agarose gel electrophoresis showing the RT PCR products of CPMV isolates coat protein gene. M: DNA Marker (1000 bp), Lanes 1 and 2: CPMV isolates 1 and 2 respectively (800 bp).
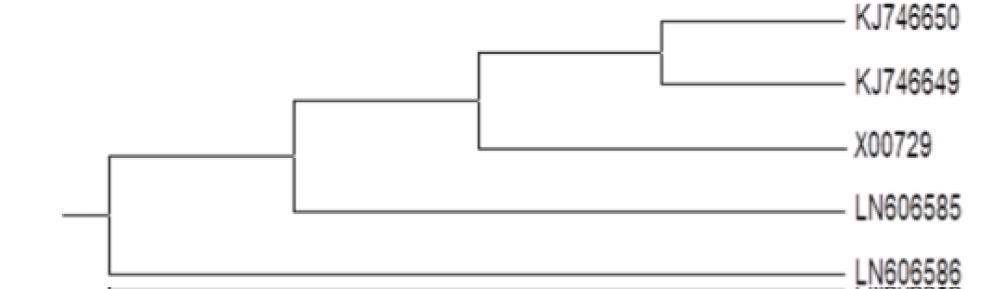
Nucleotide sequence alignment and phylogenetic analysis relationship between CPMV isolate1 and isolate2 from GenBank: Sequence of each isolates was edited using Chromas Pro Version 1.34 software, and a multiple alignment program for DNA or proteins revealed 779 (isolate1) and 772 (isolate2) nucleotides (Figure 9). The accession numbers CPMV aziz1 from El-Behera governorate was LN606585 and LN606586 for CPMVaziz2 from Alexandria governorate. Partial sequence of coat protein gene of CPMV isolate1 and isolate2 CP genes were used for comparison among previously described CPMV isolates in GenBank database using CLUSTALW program; http://www.ebi.ac.uk/clustalw [20] (Figure 10). The similarity reached 94% between our CPMV nucleotide and amino acid sequences and those of 3 CPMV isolates from GenBank (KJ746650, KJ746649 and X00729).
Figure 9: Phylogenetic tree generated using partial sequence of CPMV aziz1 (LN606585) and CPMV aziz2 (LN606586) with that of other CPMV strains obtained from GenBank based on nucleotides.
Figure 10: Sequence alignment between the two isolated CP genes from the infected cowpea plants with CPMV in El-Beheira and Alexandria. The sequence was aligned using Clustal W 1.82 program based on the obtained DNA nucleotide sequence.
Cowpea (Vigna unguiculata) is considered as one of the most economically important Fabaceae crops cultivated in different regions in Egypt. Under field conditions, cowpea plants are subjected to attack by viruses. Data concerning serological diagnosis by using indirect ELISA with specific antiserum revealed the presence of two isolates of CPMV in cowpea leaf samples collected from naturally infected cowpea plants from different regions in northern Egypt. CPMV was reported in other countries [2,6,8,9,22]. Data concerning host range revealed that CPMV isolate1 which was easily sap inoculated on cowpea cv. Kareem7) and induced chlorotic local lesions on Chenopodium amaranticolor as previously reports [6,7,9]. Symptoms of CPMV i.e. mosaic, blisters and severe distortion on Vigna unguiculata [6-9] and necrotic local lesion on inoculated leaves of Datura stramonium as same as Hampton, et al. [9]. On the contrary, faint spots appeared on inoculated primary leaves of Phaseolus vulgaris [9], necrotic local lesions on primary leaves but mild mosaic appearance on upper leaves of Glycin max [6,7,9]. The incidence of CPMV as determined in 21 day old cowpea seedlings grown from seeds collected from infected cowpea plants was as high as 65% by indirect ELISA test. Fegla, et al. [23], proved that TBIA was simpler and more practical than ELISA to be used for detection of Alfalfa mosaic alfamovirus (AMV) in flowers, pods, intact seeds and seed parts of alfalfa cv. El Wadi El-Gadid during maturation. Gilmer, et al. [24], recorded 1-5% seed transmission in cowpea in Nigeria, but Thottappilly and Rossel [25], found no evidence of seed transmission using many seeds of different cowpea varieties. Different techniques ranged from visual inspection and biological assay to immunoassays using polyclonal antiserum, or PCR can be used for viral diagnosis based on the availability, sensitivity, and inclusiveness among efficient detection techniques. The precise diagnosis is one of the keys towards virus control strategy. In our study sensitivities of indirect ELISA, TBIA and DBIA (serological tests) to detect CPMV were evaluated. It is also very important to recognize a sensitive, simple, reliable, inexpensive method for detection of the virus in the different parts of the infected plant. TBIA has advantage to be used for field surveys, including detection of virus in different plants parts. Its several advantages is such as; cheaper in cost, could be completed in less than four hours without sacrificing sensitivity, do not require sophisticated facilities, and was sensitive enough to detect the virus in all parts of infected plants [10,14-16,26]. Data concerning a possibility of using alternative solid carriers instead of nitrocellulose membrane such as canson paper (150 g/m2), is promising in TBIA. Noticeable higher signal from CPMV diseased material gave pronounced purple color by infected leaf tissues as compared to none in healthy leaf samples of cowpea. Canson paper was equally sensitive as the nitrocellulose membranes. Similar conclusions have been reported by Fegla, et al. [17] and by other investigators working with dot blot immunobinding assay (DBIA) [27-29]. Concerning the use of both faces of the nitrocellulose membrane and the canson paper as solid carriers by TBIA and gave pronounced purple color with these infected leaf tissues. Such results agreed with those reported by Al-Khalaf, et al. [30] who worked on Bean yellow mosaic virus (BYMV) and Abd El-Aziz and Youns, [14] worked on cucumber mosaic cucumovirus (CMV). TBIA was more sensitive than indirect ELISA since they detected CPMV after 2 and 4 days of inoculation, respectively. Such results however did not agreed with those reported by Abd El-Aziz, [18], Fegla, et al. [16], who found that indirect ELISA was more sensitive than DBIA with CMV and potato virus Y potyvirus (PVY). Two isolates of CPMV isolated from cowpea plants, were identified on the basis of serological reaction by indirect ELISA and by using of specific oligonucleotide primers in reverse transcription-polymerase chain reaction (RT-PCR). Our results confirmed the specificity of the primer sequence used [31-35]. The PCR product sizes were in agreement with those found by Phelps, et al. [19].
Analysis of PCR products in agarose gel electrophoresis revealed amplification of specific bands approximately 800bp for CPMV, which were in agreement with Phelps, et al. [19].
Cowpea plants under field conditions were infected with cowpea mosaic comovirus (CPMV), which caused loses in the crop. We studied the transmission and the host range which plays as an alternative host plant to avoid the increase in disease incidence. Reduction of the coast of serological tests by using a promising regular paper canson (150 g/m2) instead of NCM. Registration the isolates in GeneBank provide any investigator the map of (CPMV) distribution in Northern Egypt.
The authors briefly acknowledge those who helped them in conducting this research mainly; Prof. Gaber Ibrahim Fegla, Prof. of Plant Pathology, Faculty of Agriculture, Alexandria University; Prof. Maysa Anwar El-Said Awad, Head Research of plant pathology, Plant pathology institute, Agriculture Research Center, Giza.
- Abd El-Aziz MH. Studies on Some Viruses Infecting Cowpea Plants. Ph.D. Thesis. Fac Agric. (Saba - Basha) Alexandria University. 2015; 136.
- Thottappilly G, Rossel HW. Virus diseases of cowpea in tropical Africa. Tropical Pest Management. 1992; 38: 337-348.
- Bruening G, Agrawal HO. Infectivity of a mixture of cowpea mosaic virus ribonucleoprotein components. Virology. 1967; 32, 306-320.
- Chant SR. The effect of infection with tobacco mosaic and cowpea yellow mosaic viruses on the growth rate and yield of cowpeas in Nigeria. Imperial J Exper Agri. 1960; 28: 114120.
- Bliss FA, Robertson DG. Genetics of host reaction in cowpea to cowpea yellow mosaic virus and cowpea mottle virus. Crop Sci. 1971; 11: 258-262.
- Chant SR. Viruses of cowpea, Vigna unguiculata L. (Walp.) in Nigeria. Ann Applied Biol. 1959; 47: 565-573.
- Van Kammen A. Cowpea mosaic virus. C.M.I/A.A.B. Description of plant viruses. Kew, Surrey, England. 1971.
- Agrawal HO. Identification of cowpea mosaic virus isolates. Mededel. Landbouw. Wageningen. 1964645: 1-61.
- Hampton R, L Beczner, D Hagedorn, L Bos, T Inouye, et al. Host reactions of mechanically transmissible legume viruses of the northern temperate zone. Phytopathology. 1978; 68: 989-997.
- Younes HA, Aseel DG, Abd El-Aziz MH. Identification and purification of cowpea mosaic comovirus isolated from infected cowpea (vigna unguiculata L.) in northern Egypt. Int J Agri Environ Res. 2018; 4: 20.
- Hamza KA, Abd El-Aziz MH, Behiry SI, Younes HA. Isolation and purification of Potato virus Y isolate infecting potato (Solanum tuberosum L.) in Al-Nubaria region. Middle East J Agri Res. 2018; 7: 1201-1207.
- Powell CA. Detection of three plant viruses by dot – immunobinding assay. Phytopathology. 1987; 77: 306-309.
- Fegla GI, El-Samra IA, Younes HA, Abd El-Aziz MH. Optimization of dot immunobinding assay (DIA) for detection of tomato mosaic virus (ToMV). J Adv Agric. Res. 2000; 5:1495-1506.
- Abd El-Aziz MH, Younes HA. Detection of Cucumber mosaic cucumovirus in infected cowpea plants (Vigna unguiculata L.) from northern Egypt. Novel Res Microbiol J. 2019: 3: 326-340.
- Lin NS, Hsu YH, Hsu HT. Immunological detection of plant viruses and mycoplasmalike organism by direct tissue blotting on nitrocellulose membranes. Phytopathology. 1990; 80: 824-828.
- Fegla GI, El-Samra IA, Younes HA, Abd El-Aziz MH. Comparative studies for detection of Tomato Mosaic Tobamovirus (ToMV), Cucumber Mosaic Cucumovirus (CMV) and Potato Y Potyviruses (PVY). Adv Agric Res. 2001a; 6: 239-254.
- Fegla0 GI, El-Samra IA, Younes HA, Abd El-Aziz MH. Plain and Filter papers as solid carriers for detection of three plant viruses by dot and tissue blot immunoassays. Adv Agric Res. 2001b; 6: 755-761.
- Abd El-Aziz MH. Detection of certain plant viruses. Ms. C. Thesis. Fac. Of Agric. (Saba–Basha) Alex. Univ. Egypt. 2000; 118.
- Phelps JP, Dang N, Rasochova. Inactivation and purification of cowpea mosaic virus like particles displaying peptide antigens from Bacillus anthracis. J Virol Methods. 2007; 141: 146-153.
- Thompson JD, Higgins DG, Gibson TJ. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994; 22: 4673-4680. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7984417
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004; 5: 150-163. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15260895
- Laughlin M, Pornpod MR, Thongmee A, Milbrath GM, Goodman RM. Isolation and some properties of a yellow subgroup member of cowpea mosaic virus from Illinois. Phytopathology. 1977; 67: 844-847.
- Fegla GI, Fath-Allah MM, Younes HA. Alfalfa mosaic alfamovirus in alfalfa floral parts, pods and seeds at different stages of development. J Agric Sci. Mansoura Univ. 2004; 29: 4931-4939.
- Gilmer RM, Whitney WK, Williams RJ. Epidemiology and control of cowpea mosaic in western Nigeria. In Proceedings of First IITA Grain Legume Workshop. IITA Ibadan, Nigeria. 1974.
- Thottappilly G, Rossel HW. Seed transmission of cowpea yellow mosaic virus unlikely in cowpea. Tropical Grain Legume Bulletin. 1988; 34: 27-28.
- Makkouk KM, Kumari SG. Detection of ten viruses by the tissue-blot immunoassay (TBIA). Arab Journal of Plant Protection. 1996; 14: 3-9.
- Sherwood JL. Comparison of a filter paper immunobinding assay, western blotting and an enzymelinked immunosorbent assay for the detection of wheat streak mosaic virus. J Phytopathology. 1987; 118: 6875.
- Heide M, Lang L. Detection of Potato leaf roll virus and potato viruses M, S, X and Y by dot immunobinding on plain paper. Potato Research. 1988; 31: 367-373.
- Lange L, Jomantor A, Heide M. Testing seeds for viruses by dot immunobinding (DIB) directly on plain paper. Tidsskrift-for-Planteavl. 1989; 93: 93-96.
- Al Khalaf M, Kumari SG, Haj Kassem AA, Makkouk KM, Al Chaabi S. Use of the two faces of nitrocellulose membrane in tissue blot immunoassay for the detection of Bean yellow mosaic virus and the possibility of its mechanically transmitted from the printed membrane to the host plant. Arab J Plant Protection. 2009; 27: 91-94.
- Hadidi A, Levy L, Podieckis V. Polymerase chain reaction technology in plant pathology. In: Molecular methods in Plant Pathol. Singh RP, Singh US. (eds.) CRC press. Boca Raton, FL. 1995; 167-187.
- Hsu HT, Lawson RH. Direct tissue blotting for detection of tomato spotted wilt virus in Impatiens. Plant Disease. 1991; 75: 292-295.
- Klein RE, Wyatt SD, Kaiser WJ, Mink GI. Comparative immunoassays of bean common mosaic virus in individual bean (Phaseolus vulgaris) seed and bulked bean seed samples. Plant Dis. 1992; 76:57-69.
- Lizarraga C, Fernandez-Northcote EN. Detection of Potato Viruses X and Y in Sap Extracts by a Modified Indirect Enzyme-Linked Immunosorbent Assay on Nitrocellulose Membranes (NCM ELISA). Plant Dis. 1989; 73: 11-14.
- Thompson KG, Dietzgen RG, Gibbs AJ, Tang YC, Liesack W, et al. Identification of Zucchini yellow mosaic virus by RT-PCR and analysis of sequence variability. J Virol Methods. 1995; 55:83-96. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8576311